单宁酸可注射水凝胶抗铁下垂促进外伤性脑损伤恢复
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
创伤性脑损伤(TBI)可引发一系列复杂的生理反应,其中铁超载和神经元铁下垂起着尤为关键的作用。这些过程加重了继发性脑损伤,并显著恶化神经功能。为了应对这一挑战,本研究开发了一种创新的局部给药策略:一种可注射的创伤后微环境反应水凝胶。由单宁酸(TA)和季铵化壳聚糖(QCS)组成的水凝胶通过其抗铁下沉机制缓解脑外伤后继发性脑损伤的神经功能缺损。在体外,用铁下垂诱导剂RSL-3处理HT22细胞建立铁下垂模型,证明水凝胶在tbi样条件下具有抗氧化能力。结果表明,水凝胶能明显恢复细胞活力,逆转铁积累,减轻脂质过氧化,恢复线粒体功能。进一步的TBI模型体内实验表明,TA/QCS水凝胶不仅能有效抑制神经元变性、减少铁积累和脂质过氧化,还能恢复神经元的线粒体功能。此外,水凝胶通过抑制小胶质细胞和星形胶质细胞的激活来显著减轻神经炎症,从而促进TBI后神经系统的恢复。这项研究为TBI管理策略提供了新的见解,旨在防止继发性损伤的进展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
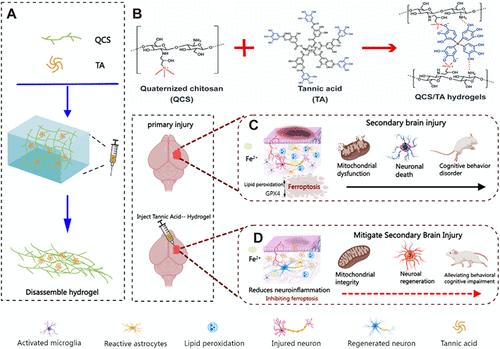
Tannic Acid-Based Injectable Hydrogel Promotes the Recovery of Traumatic Brain Injury by Anti-Ferroptosis
Traumatic brain injury (TBI) can trigger a series of complex physiological responses, with iron overload and neuronal ferroptosis playing particularly pivotal roles. These processes exacerbate secondary brain injury and significantly deteriorate neurological function. To address this challenge, this study developed an innovative local drug delivery strategy: an injectable, post-traumatic microenvironment-responsive hydrogel. The hydrogel, composed of tannic acid (TA) and quaternized chitosan (QCS), is designed to alleviate neurological deficits secondary brain injury following TBI through its anti-ferroptosis mechanism. In vitro, a ferroptosis model was established using HT22 cells treated with the ferroptosis inducer RSL-3, demonstrating the hydrogel’s antioxidant capacity in the TBI-like conditions. The results showed that the hydrogel significantly restored cell viability, reversed iron accumulation, alleviated lipid peroxidation, and restored mitochondrial function. Further in vivo experiments in the TBI model showed that the TA/QCS hydrogel not only effectively inhibited neuronal degeneration, reduced iron accumulation, and lipid peroxidation but also restored mitochondrial function in neurons. Additionally, the hydrogel significantly attenuated neuroinflammation by inhibiting the activation of microglia and astrocytes, thereby facilitating neurological recovery after TBI. This study offers novel insights into TBI management strategies aimed at preventing the progression of a secondary injury.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


