抗肿瘤缺氧和抗氧化微环境的不依赖氧治疗纳米系统
IF 3.7
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Journal of photochemistry and photobiology. B, Biology
Pub Date : 2025-05-18
DOI:10.1016/j.jphotobiol.2025.113184
引用次数: 0
摘要
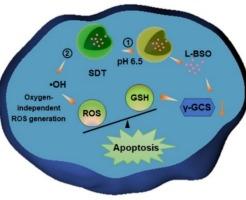
先天缺氧和抗氧化被告微环境是提高活性氧(ROS)抗癌疗效的主要障碍。本文制备了钨酸铋纳米粒子(NPs),利用其价带位置的正电位比OH•的生成电位更强,通过与水的不依赖氧的反应产生超声激活羟基自由基(OH•)。为了缓解抗氧化防御,设计了通过在空心BWO NPs中加载l -丁硫氨酸亚砜胺来抑制谷胱甘肽(GSH)的合成,并在表面包裹ph响应的单宁酸-铁络合物来防止药物泄漏的BWOST NPs。体外和体内实验均表明,BWOST NPs能有效降低细胞内GSH水平,诱导癌细胞凋亡,消除肿瘤。因此,BWOST NPs通过降低抗氧化防御和不依赖氧的OH•生成,显示出放大的声动力治疗效果,这为改善基于ROS的癌症治疗提供了有效的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
An oxygen-independent therapeutic nanosystem for fighting against hypoxic and antioxidant microenvironment of tumor
The innate hypoxic and antioxidant defendant microenvironment are the main obstacles for improving reactive oxygen species (ROS) based therapeutic efficacy against cancer. Herein, bismuth tungstate (BWO) nanoparticles (NPs) were fabricated for ultrasound activated hydroxyl radical (OH•) production through being reacted with water in an oxygen-independent manner due to their more positive potential of valent band position than that of OH• generation. For relieving antioxidant defense, BWOST NPs were designed through loading L-buthionine sulfoximine into hollow BWO NPs to inhibit the synthesis of glutathione (GSH), and coating pH-responsive tannic acid-Fe complex on the surface to prevent drug leakage. Both in vitro and in vivo assessments demonstrated that BWOST NPs could effectively lower intracellular GSH levels, inducing apoptosis of cancer cells and eliminating tumors. Therefore, BWOST NPs showed an amplified sonodynamic therapy efficacy through lower antioxidant defense and oxygen-independent OH• generation, which provided an effective strategy for improving ROS based cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
12.10
自引率
1.90%
发文量
161
审稿时长
37 days
期刊介绍:
The Journal of Photochemistry and Photobiology B: Biology provides a forum for the publication of papers relating to the various aspects of photobiology, as well as a means for communication in this multidisciplinary field.
The scope includes:
- Bioluminescence
- Chronobiology
- DNA repair
- Environmental photobiology
- Nanotechnology in photobiology
- Photocarcinogenesis
- Photochemistry of biomolecules
- Photodynamic therapy
- Photomedicine
- Photomorphogenesis
- Photomovement
- Photoreception
- Photosensitization
- Photosynthesis
- Phototechnology
- Spectroscopy of biological systems
- UV and visible radiation effects and vision.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


