局部微环境和表面动力学对Cu(100)单晶电极上C2H4选择性影响的研究
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
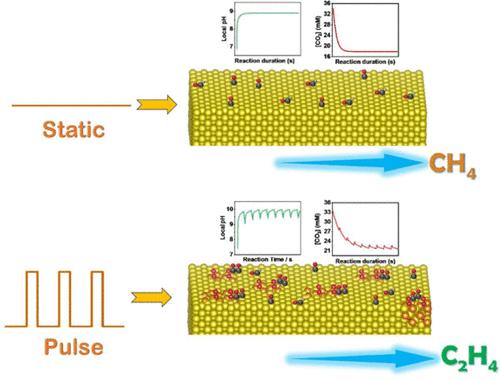
电化学还原CO2 (eCO2RR)用于铜上的C2H4生产,是在生产燃料和增值化学原料时减少温室气体排放的一种有前途的方法。了解eCO2RR机制并确定影响其选择性的因素对于设计有效的电化学系统至关重要。然而,大多数文献中使用的多晶铜衬底/颗粒使得研究单一因素的影响具有挑战性。本文制备了暴露于(100)表面的单晶薄膜,并利用它来探索C2H4和CH4之间选择性切换的潜在机制。微环境的演化,特别是局部的pH和CO2浓度,被推测是C2H4对Cu(100)选择性变化的主要原因。考虑到这一猜想,采用脉冲电位策略,通过调整阳极脉冲宽度来精细调节电化学系统的局部pH值。基于局部pH演化和operando拉曼测量的模拟结果,Cu表面的局部pH效应和化学状态是脉冲增强C2H4选择性和C2H4/CH4对脉冲宽度的火山状依赖的原因。C2H4的最高法拉第效率(FE)为51.75%,C2的最大选择性为65.5%。这项工作可能为电催化系统中产物选择性的调制提供见解,而不仅仅局限于eCO2RR。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Insights into the Influence of Local Microenvironment and Surface Dynamics on C2H4 Selectivity over Cu(100) Single-Crystal Electrode
Electrochemical reduction of CO2 (eCO2RR) for C2H4 production over Cu presents a promising approach for mitigating greenhouse gas emissions while producing fuels and value-added chemical feedstocks. Understanding the eCO2RR mechanism and identifying the factors that can affect its selectivity are essential for the design of effective electrochemical systems. However, the polycrystalline Cu substrates/particles used in most literature make it challenging to investigate the influence of a single factor. Herein, single-crystalline foils exposing the (100) surface are prepared and employed to explore the underlying mechanism of the selectivity switch between C2H4 and CH4. Evolution in the microenvironment, especially the local pH and CO2 concentration, is speculated to be the main cause of the observed selectivity change from C2H4 to CH4 over Cu(100). Considering this conjecture, a pulsed potential strategy is used to finely modulate the local pH of the electrochemical system via tuning the anodic pulse width. Based on the results of simulations for local pH evolution and operando Raman measurements, the local pH effect and chemical states on the Cu surface are responsible for the pulse-enhanced C2H4 selectivity and the volcano-shaped dependence of C2H4/CH4 on pulse width. The highest C2H4 Faradaic efficiency (FE) reaches 51.75%, with the maximum C2 selectivity of 65.5%. This work may provide insights into the modulation of product selectivity in electrocatalytic systems, not just limited to eCO2RR.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


