5-乙基-2-降冰片烯(ENB)和5-乙烯基-2-降冰片烯(VNB)基脂环多元醇合成聚酯
IF 3.9
2区 化学
Q2 POLYMER SCIENCE
引用次数: 0
摘要
具有高玻璃化转变温度的刚性脂环单体衍生出的非晶态聚酯作为聚碳酸酯的潜在替代品而备受关注。因此,5-乙基-2-降冰片烯(ENB)和5-乙烯基-2-降冰片烯(VNB)被认为是提高聚酯热性能的二醇单体前驱体。对ENB和VNB的区域选择性合成相应的二醇进行了优化,并进行了首次规模实验,为这些聚酯单体的大规模生产提供了有希望的结果。此外,为了证明概念,建立了一种合成纯支化二醇的替代方法。以对苯二甲酸二甲酯(DMT)为模型化合物进行了聚合实验,并与常用的聚对苯二甲酸乙酯进行了比较。因此,制备了一系列含有支链或线性或两者混合区域异构体的聚酯,并通过GPC, TGA和DSC分析研究了结构性能关系。Tg值的变化范围为75 ~ 103℃,这取决于聚酯微观结构中的支链部分和线性部分及其分子量。此外,异山梨酯(IS)作为生物基共聚物被引入,得到了无定形共聚酯,Tgs范围为81 ~ 97°C。本文章由计算机程序翻译,如有差异,请以英文原文为准。
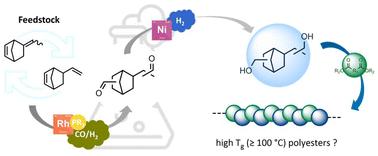
5-Ethylidene-2-norbornene (ENB) and 5-vinyl-2-norbornene (VNB) based alicyclic polyols for the synthesis of polyesters†
Amorphous polyesters derived from rigid alicyclic monomers with high glass transition temperatures are of great interest as potential substitutes for poly(carbonate)s. Therefore, 5-ethylidene-2-norbornene (ENB) and 5-vinyl-2-norbornene (VNB) were identified as interesting diol monomer precursors to enhance the thermal properties of polyesters. The regioselective synthesis of the corresponding diols from ENB and VNB was optimized and the first scale-up experiments gave promising results for a potential large-scale production of these polyester monomers. Moreover, for proof of concept, an alternative procedure was established to synthesize exclusively branched diols. Polymerization experiments were conducted with dimethyl terephthalate (DMT) as a model compound to compare the polyesters with commonly used poly(ethylene terephthalate)s. Therefore, a series of polyesters containing branched or linear regioisomers or a mixture of both regioisomers were produced, and structure–property relationships were investigated using GPC, TGA and DSC analyses. The Tg values ranged from 75 to 103 °C, depending on the branched and linear moieties in the polyester microstructure and their molecular weights. Moreover, isosorbide (IS) was introduced as a biobased comonomer, resulting in amorphous copolyesters with promising Tgs ranging from 81 to 97 °C.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Chemistry
POLYMER SCIENCE-
CiteScore
8.60
自引率
8.70%
发文量
535
审稿时长
1.7 months
期刊介绍:
Polymer Chemistry welcomes submissions in all areas of polymer science that have a strong focus on macromolecular chemistry. Manuscripts may cover a broad range of fields, yet no direct application focus is required.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


