外源添加重组CLIC蛋白对培养细胞具有抗氧化保护作用
IF 2.7
Advances in redox research : an official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe
Pub Date : 2025-05-16
DOI:10.1016/j.arres.2025.100132
引用次数: 0
摘要
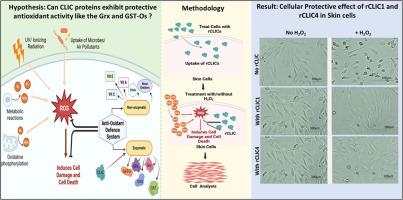
细胞内氯离子通道(CLICs)是一个由六种人类蛋白组成的蛋白家族,它们以可溶性和整体膜蛋白的形式存在,并在全身不同组织中表达。CLIC1和CLIC4作为兼职蛋白,除了具有膜离子通道活性外,还具有氧化还原酶活性。在原代人真皮成纤维细胞(HDF)、人表皮角质形成细胞(HKE)细胞和稳定的小鼠成纤维细胞系NIH/3T3中,瞬时siRNA敲低CLIC1或CLIC4均显示细胞活力显著降低。相反,过表达CLIC1或CLIC4的NIH/3T3细胞表明,这两种蛋白有助于保护细胞免受过氧化氢(H2O2)诱导的氧化损伤,从而减少细胞死亡和活性氧(ROS)的产生。而在使用siRNA沉默这些蛋白质的细胞中,则出现了相反的效果。我们现在也证明,通过外源性添加重组CLIC (rCLIC)蛋白到培养的HDF或HKE细胞中,rCLIC1和rCLIC4蛋白都能提供细胞抗氧化保护,使成纤维细胞和角质细胞免受h2o2诱导的氧化损伤。我们的研究还证明了rCLIC1和rCLIC4作为皮肤细胞保护抗氧化剂的能力,这源于它们的氧化还原酶酶活性。我们的研究结果还表明,与其他众所周知的内源性抗氧化剂如谷胱甘肽(Grx)、谷胱甘肽s -转移酶- omega (GST-Ω)和抗氧化药物n -乙酰半胱氨酸(NAC)相比,外源性添加rCLIC1或rCLIC4到皮肤细胞对h2o2诱导的氧化损伤具有相似或更强的保护作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exogenously added recombinant CLIC proteins provide antioxidant protection to cells in culture
Chloride intracellular ion channels (CLICs) are a family of six human proteins that exist as both soluble and integral membrane proteins and are expressed across a range of different tissues throughout the body. CLIC1 and CLIC4 act as moonlighting proteins, exhibiting oxidoreductase enzymatic activity in addition to their membrane ion channel activity. Transient siRNA knockdown of either CLIC1 or CLIC4 in primary human dermal fibroblast (HDF), human epidermal keratinocyte (HKE) cells and in the stable murine fibroblast cell line, NIH/3T3, showed significant reduction in cell viability. Conversely, NIH/3T3 cells over-expressing CLIC1 or CLIC4 demonstrated that both proteins assist in protecting the cells from hydrogen peroxide (H2O2)-induced oxidative damage, resulting in reduced cell death and reduced Reactive Oxygen Species (ROS) generation. While the opposite effect was seen in cells where these proteins had been silenced using siRNA. We have also now demonstrated that by exogenously adding recombinant CLIC (rCLIC) proteins to either HDF or HKE cells in culture, both rCLIC1 and rCLIC4 proteins provided cellular antioxidant protection to the fibroblast and keratinocyte cells against H2O2-induced oxidative damage. Our study also demonstrates rCLIC1 and rCLIC4’s ability to act as skin cell protective antioxidant agents, arises from their oxidoreductase enzymatic activity. Our findings also showed exogenous addition of rCLIC1 or rCLIC4 to skin cells resulted in similar or greater protection against H2O2-induced oxidative damage when compared to other well-known endogenous antioxidants like glutaredoxin (Grx), Glutathione S-transferase-Omega (GST-Ω) and the antioxidant drug, N-acetylcysteine (NAC).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advances in redox research : an official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe
Biochemistry, Genetics and Molecular Biology (General)
CiteScore
2.60
自引率
0.00%
发文量
0
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


