用ipso-碳亲核试剂催化烯烃不对称亲电氯碳环化
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
烯烃催化不对称亲电双官能化是获得对映体富集化合物的有力策略。尽管在这一领域取得了重大进展,但用ipso-碳亲核试剂实现这种转化仍然是一个艰巨的挑战。在此,我们报道了一种前所未有的方法,使用ipso-碳亲核试剂,使用n -氯丁二酰亚胺(NCS)作为亲电试剂和大体积手性硫化物催化剂,实现烯烃的不对称亲电双官能化。以1,1-二取代烯烃为底物,以苯酚衍生的芳基为亲核试剂,合成了一系列手性吡咯烷类化合物,含两个全碳季立体中心和一个螺环己二酮部分,收率高,对映选择性高。此外,该反应通过原位脱芳-再芳化工艺扩展到手性四氢苯并恶氮卓类和二乙基胺的合成。在这些转化中,立体化学控制完全通过路易斯碱络合来实现,而不需要辅助的结合相互作用。对照实验表明,亲电试剂的选择和底物上磺酰基的构象控制对反应的有效进行至关重要。密度泛函理论(DFT)研究表明,亲电氯与催化剂硫原子上的两个孤对之一的选择性配位具有能量偏好,形成手性硫中间体,随后对烯烃π键进行面部识别。本文章由计算机程序翻译,如有差异,请以英文原文为准。
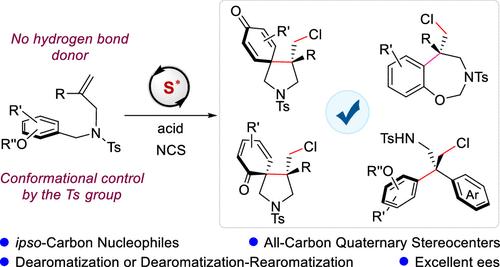
Catalytic Asymmetric Electrophilic Chlorocarbocyclization of Alkenes Using ipso-Carbon Nucleophiles
Catalytic asymmetric electrophilic difunctionalization of alkenes is a powerful strategy for accessing enantioenriched compounds. Despite significant progress in this field, achieving such transformations with ipso-carbon nucleophiles remains a formidable challenge. Herein, we report an unprecedented method for the asymmetric electrophilic difunctionalization of alkenes using ipso-carbon nucleophiles, employing N-chlorosuccinimide (NCS) as the electrophilic reagent and a bulky chiral sulfide catalyst. By utilizing 1,1-disubstituted alkenes as substrates and tethered phenol-derived aryl groups as ipso-carbon nucleophiles, a series of chiral pyrrolidines bearing up to two all-carbon quaternary stereocenters and a spirocyclohexadienone moiety were synthesized in good yields with high enantioselectivities. Furthermore, this reaction was extended to the synthesis of chiral tetrahydrobenzoxazepines and diarylethylamines via an in situ dearomatization-rearomatization process. In these transformations, stereochemical control is achieved exclusively through Lewis base complexation without the need for auxiliary binding interactions. Control experiments revealed that both the choice of electrophilic reagents and the conformational control imparted by the sulfonyl protecting group on the substrates are critical for the efficient progression of the reaction. Density functional theory (DFT) studies demonstrated an energetic preference for the selective coordination of electrophilic chlorine to one of the two lone pairs on the sulfur atom of the catalyst, forming a chiral sulfur intermediate, followed by facial recognition of the alkene π bonds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


