仿生聚苯胺膜对tau蛋白磷酸化水平的电化学调节
IF 4.9
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
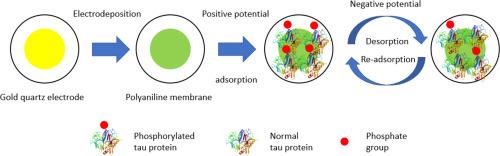
阿尔茨海默病(AD)的特点是过度磷酸化的tau蛋白积累,这会破坏微管稳定性,并显著导致神经变性和认知能力下降。尽管现有的治疗方法针对tau病理,但其疗效仍然有限,强调迫切需要创新的治疗方法。研究了一种利用导电聚苯胺(PANI)膜有效调节tau蛋白磷酸化水平的新电化学方法。逆转tau蛋白过度磷酸化为阿尔茨海默病提供了一种突破性的治疗策略,为更有效的治疗方式和改善患者预后开辟了新的途径。研究表明,在静电刺激下,磷酸化的tau肽和蛋白可以有效地粘附在聚苯胺膜上。AFM分析结果显示,裸电极和tau蛋白/聚苯胺修饰电极之间的表面形态发生了变化,表明聚苯胺形成了一个均匀、光滑的膜,通过正电位而不是蛋白质自聚集吸引p-tau。pS214肽在0.5 V和磷酸化tau蛋白在0.3 V时的最佳吸附电位被确定。电化学石英晶体微天平(EQCM)测量显示,负电位下的最大吸附效率为40%,解吸效率为16.67%。此外,交替的正负电位可以重复和精确地控制tau附着和脱离聚苯胺膜。通过质谱法验证了这种动态调节,证实了在此过程中磷酸基团的去除。提出的电化学方法提供了一种精确的方法来控制tau肽和蛋白质中的磷酸基团浓度。这种精确性支持生物相容性植入物的发展,可能有助于治疗神经退行性疾病。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electrochemical modulation of tau protein phosphorylation levels with biomimetic polyaniline membranes
Alzheimer's disease (AD) is hallmarked by the accumulation of hyperphosphorylated tau proteins, which disrupt microtubule stability and significantly contribute to neurodegeneration and cognitive decline. Despite existing therapies targeting tau pathologies, their efficacy remains limited, underscoring the urgent need for innovative therapeutic approaches. A novel electrochemical method utilizing conductive polyaniline (PANI) membranes to effectively regulate tau protein phosphorylation levels was investigated. Reversing tau hyperphosphorylation provides a groundbreaking therapeutic strategy for AD, opening new avenues for more effective treatment modalities and improving patient outcomes.
The research demonstrates that phosphorylated tau peptides and proteins adhere effectively to PANI membranes under electrostatic stimulation. The results of AFM analysis revealed variation in surface morphology between the bare electrode and the tau protein/PANI-modified electrode, indicating that PANI forms an even, smooth membrane that attracts p-tau through positive potential rather than protein self-aggregation. Optimal adsorption potentials were identified at 0.5 V for pS214 peptides and 0.3 V for phosphorylated tau proteins. Electrochemical quartz crystal microbalance (EQCM) measurements showcased a maximum adsorption efficiency of 40 %, with desorption efficiencies reaching 16.67 % at negative potentials. Furthermore, alternating positive and negative potentials enabled repeated and precise control over tau attachment and detachment from the PANI membranes. This dynamic regulation was verified through mass spectrometry, which confirmed the removal of phosphate groups during the process.
The proposed electrochemical approach offers a precise method for controlling phosphate group concentrations in tau peptides and proteins. This precision supports the development of biocompatible implants that could be instrumental in treating neurodegenerative diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sensing and Bio-Sensing Research
Engineering-Electrical and Electronic Engineering
CiteScore
10.70
自引率
3.80%
发文量
68
审稿时长
87 days
期刊介绍:
Sensing and Bio-Sensing Research is an open access journal dedicated to the research, design, development, and application of bio-sensing and sensing technologies. The editors will accept research papers, reviews, field trials, and validation studies that are of significant relevance. These submissions should describe new concepts, enhance understanding of the field, or offer insights into the practical application, manufacturing, and commercialization of bio-sensing and sensing technologies.
The journal covers a wide range of topics, including sensing principles and mechanisms, new materials development for transducers and recognition components, fabrication technology, and various types of sensors such as optical, electrochemical, mass-sensitive, gas, biosensors, and more. It also includes environmental, process control, and biomedical applications, signal processing, chemometrics, optoelectronic, mechanical, thermal, and magnetic sensors, as well as interface electronics. Additionally, it covers sensor systems and applications, µTAS (Micro Total Analysis Systems), development of solid-state devices for transducing physical signals, and analytical devices incorporating biological materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


