MCSP+转移创始细胞在人类黑色素瘤转移定植早期激活免疫抑制。
IF 28.5
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
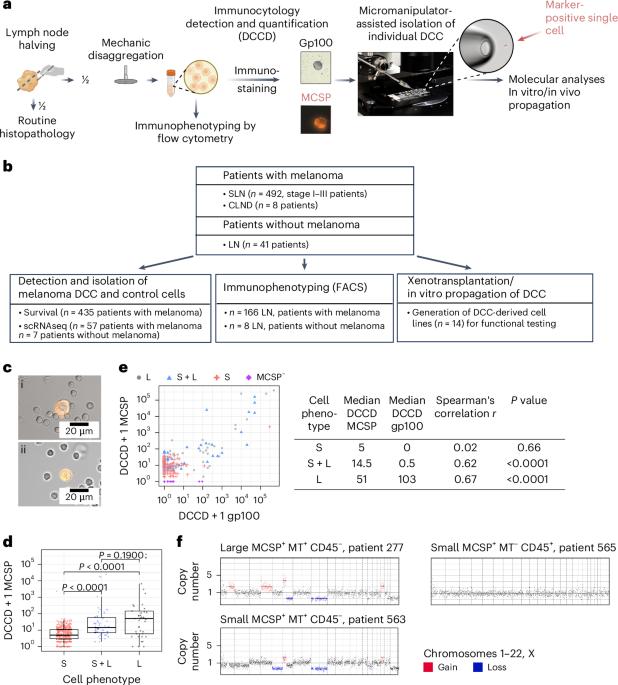
为了研究早期,知之甚少的事件驱动转移进展,我们在492例I-III期黑色素瘤前哨淋巴结(SLN)活检中寻找最早可检测到的播散性癌细胞(DCCs),也通常被称为播散性肿瘤细胞(dtc)。通过微操作仪辅助分离罕见的dcc,单细胞mRNA和DNA测序,免疫荧光成像和生存分析,我们确定黑色素瘤相关硫酸软骨素蛋白多糖(MCSP)+黑色素瘤细胞为转移建立细胞(mfc)。我们发现进入sln的dcc主要表现出一种短暂的表型,在CD8 T细胞触发干扰素γ暴露后,去分化为神经冠样表型。这伴随着携带免疫调节蛋白CD155和CD276的小细胞外囊泡(sev)的增加,但很少携带程序性细胞死亡蛋白1配体1。sev抑制CD8 T细胞增殖和功能,促进集落形成。靶向MCSP+ mfc或其免疫逃逸机制可能是通过预防转移表现而早期治疗黑色素瘤的关键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
MCSP+ metastasis founder cells activate immunosuppression early in human melanoma metastatic colonization
To investigate the early, poorly understood events driving metastatic progression, we searched for the earliest detectable disseminated cancer cells (DCCs), also often referred to as disseminated tumor cells (DTCs), in sentinel lymph node (SLN) biopsies of 492 patients with stage I–III melanoma. Using micromanipulator-assisted isolation of rare DCCs, single-cell mRNA and DNA sequencing, codetection by indexing immunofluorescence imaging and survival analysis, we identified melanoma-associated chondroitin sulfate proteoglycan (MCSP)+ melanoma cells as metastasis founder cells (MFCs). We found that DCCs entering SLNs predominantly exhibited a transitory phenotype that, upon interferon-γ exposure triggered by CD8 T cells, dedifferentiated into a neural-crest-like phenotype. This was accompanied by increased production of small extracellular vesicles (sEVs) carrying the immunomodulatory proteins CD155 and CD276 but rarely programmed cell death protein 1 ligand 1. The sEVs suppressed CD8 T cell proliferation and function, facilitating colony formation. Targeting MCSP+ MFCs or their immune escape mechanisms could be key to curing melanoma early by preventing manifestation of metastasis. Guetter et al. identify a melanoma-associated chondroitin sulfate proteoglycan-positive subpopulation of metastasis founder cells from lymph node biopsies of patients with melanoma and observe that they mediate immune evasion and predict systemic metastasis and poor outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature cancer
Medicine-Oncology
CiteScore
31.10
自引率
1.80%
发文量
129
期刊介绍:
Cancer is a devastating disease responsible for millions of deaths worldwide. However, many of these deaths could be prevented with improved prevention and treatment strategies. To achieve this, it is crucial to focus on accurate diagnosis, effective treatment methods, and understanding the socioeconomic factors that influence cancer rates.
Nature Cancer aims to serve as a unique platform for sharing the latest advancements in cancer research across various scientific fields, encompassing life sciences, physical sciences, applied sciences, and social sciences. The journal is particularly interested in fundamental research that enhances our understanding of tumor development and progression, as well as research that translates this knowledge into clinical applications through innovative diagnostic and therapeutic approaches. Additionally, Nature Cancer welcomes clinical studies that inform cancer diagnosis, treatment, and prevention, along with contributions exploring the societal impact of cancer on a global scale.
In addition to publishing original research, Nature Cancer will feature Comments, Reviews, News & Views, Features, and Correspondence that hold significant value for the diverse field of cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


