类金属元素和非金属元素对高熵合金不同掺杂强化效应的物理机制——以硼和碳为例
IF 14.3
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
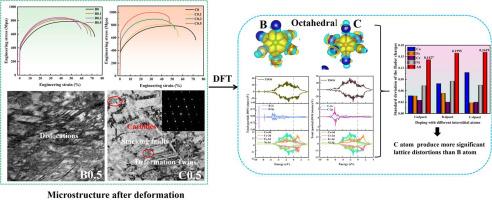
本文选择硼(B)和碳(C)分别作为类金属元素和非金属元素的代表。将它们掺杂到典型的高熵合金(HEA) Co35Cr25Fe20Ni20中,研究它们不同的增韧效果。拉伸结果证实,B和C原子的掺杂均能明显提高屈服强度和拉伸强度,且C的强化效果强于B。造成两者强化效果不同的明显原因如下:电子定位函数(ELF)表明C比B产生更明显的共价键特征,B更倾向于与Fe原子形成共价键。而C更容易与Cr原子形成共价键,这就解释了Cr23C6碳化物的形成。虽然B原子和C原子在HEA体系中都以八面体(OCT)间隙存在,但它们的贝德电荷总体转移的标准误差(SD)表明,C原子掺杂比B原子掺杂产生更大的原子水平应力。这意味着,C原子掺杂引起更明显的局域晶格畸变,产生比b原子掺杂更强的强化效应。由此可见,B等类金属元素的强化机制除了晶界强化外,主要是位错强化,而C等非金属元素的强化机制主要是晶格摩擦应力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Physical mechanisms behind the different doping strengthening effects of metalloid and non-metallic elements on high-entropy alloys: Taking boron and carbon as examples
In this paper, boron (B) and carbon (C) are chosen as the representatives of metalloid and non-metallic elements, respectively. They were doped into a typical high-entropy alloy (HEA) Co35Cr25Fe20Ni20, to study their different strengthening-toughening effects. Tensile results confirmed that the doping of both B and C atoms can obviously increase the yield and tensile strengths, and the strengthening effect of C is stronger than that of B. The apparent reasons for their different strengthening effects are as follows. The electron localization function (ELF) shows that C is to produce more pronounced covalent bonding features than B. B is more inclined to form covalent bonds with Fe atoms. Whereas C is more likely to form covalent bonds with Cr atoms, which explains the formation of Cr23C6 carbides. Although both B and C atoms exist as octahedral (OCT) interstitials in the HEA system, their standard errors (SD) of the overall transfer of Bader's charge demonstrate that the C-atom doping generates a greater atomic-level stress than the B atoms. That means, the C atoms doping causes more pronounced localized lattice distortion, producing a stronger strengthening effect than that of B-atom doping. Thus, apart from grain boundary strengthening, the strengthening mechanism of doping metalloid elements like B is mainly dislocation strengthening, but is chiefly lattice friction stress for non-metallic elements like C.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Materials Science & Technology
工程技术-材料科学:综合
CiteScore
20.00
自引率
11.00%
发文量
995
审稿时长
13 days
期刊介绍:
Journal of Materials Science & Technology strives to promote global collaboration in the field of materials science and technology. It primarily publishes original research papers, invited review articles, letters, research notes, and summaries of scientific achievements. The journal covers a wide range of materials science and technology topics, including metallic materials, inorganic nonmetallic materials, and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


