具有过量羟基的共价有机框架,一个准备磷酸化的平台,然后参与高酸度下的铀吸附
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
固相萃取法在高酸性条件下回收铀受到吸附剂的限制。共价有机框架(COFs)是一种很有前途的吸附剂。然而,大多数COF吸附剂在高酸性环境下使用时存在萃取效率低或结构分解的问题。本文介绍了COF-Tris-P作为高酸性条件下回收铀的优良吸附剂。COF-Tris- p的优异性能可归因于其前体COF-Tris遗传的高稳定性以及COF-Tris磷酸化后其孔内大量的磷酸基团。吸附实验表明,COF-Tris-P对铀酰离子的吸附量随着酸度的增加而逐渐减小,但随着酸度的进一步增加而略有反弹。令人鼓舞的是,在2 mol/L HNO3溶液中,饱和吸附容量仍然达到112.8 mg/g。同时,采用一致的协议合成了COF-EAA-P作为COF-Tris-P的类似物。COF-EAA-P在不同酸度下的吸附量与COF-Tris-P的趋势相同,只是COF-EAA-P的吸附量总体较低。相比之下,COF-Tris- p表现更好,因为COF-Tris携带大量羟基,这增强了COF-Tris- p上特定官能团的数量/密度的后修饰。本研究为COFs的结构设计和应用研究提供了一种创新思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
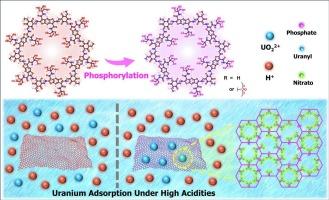
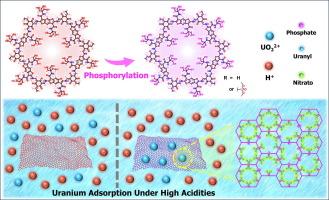
Covalent organic framework with excessive hydroxyl groups, a platform ready for phosphorylation then involved in uranium adsorption under high acidity
The uranium recovery under high acidities by solid phase extraction is constrained by the adsorbents. Covalent organic frameworks (COFs) have been promising adsorbents. However, the majority of COF adsorbents suffer from low extraction efficiencies or structural decomposition when put into use under highly acidic environments. Here we present COF-Tris-P as an excellent adsorbent for uranium recovery under high acidities. The remarkable performance of COF-Tris-P can be attributed to the high stability inherited from the precursor COF-Tris and considerable amount of phosphate groups within its pores after phosphorylation of COF-Tris. Adsorption experiments showed the adsorption amounts of COF-Tris-P for uranyl ions decrease gradually with the increase of acidities, but slightly rebound when the acidity further increased. Encouragingly, the saturated adsorption capacity still reaches 112.8 mg/g in 2 mol/L HNO3 solutions. Meanwhile, COF-EAA-P was synthesized as the analogue for COF-Tris-P employing a consistent protocol. COF-EAA-P showed the same trend as COF-Tris-P in adsorption amounts at different acidities, only the adsorption amounts of COF-EAA-P were lower overall. By comparison, COF-Tris-P outperforms because COF-Tris carries massive hydroxyl groups, which enhance the post-modification for increased amount/density of specific functional groups on COF-Tris-P. This work offers an innovative approach to the structural design and applied research of COFs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


