纳米尺度下粘土表面铀的配位:EXAFS数据和从头算分子动力学的集成
IF 5.8
2区 地球科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
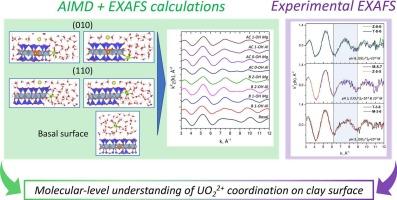
在分子水平上了解铀和粘土矿物之间的相互作用对于解决环境污染和核废料管理至关重要。在这项工作中,我们的重点是利用EXAFS光谱和从头算分子动力学模拟(AIMD)相结合的方法确定蒙脱石上铀(VI)表面物质的分子水平结构。用铀酰阳离子吸附在3种不同pH、不同铀浓度的膨润土上制备了一组样品。为了阐明黏土矿物基表面和边缘位置的铀酰配位模型在纳米尺度上的结构,对黏土矿物基表面和边缘位置的铀酰配位模型进行了AIMD模拟。实验EXAFS光谱与AIMD轨迹计算的线性组合拟合使我们能够指定pH值为3、5和8时每种吸附复合物构型的分数。在低pH条件下,铀酰阳离子的吸附形态主要为外球基配合物。pH为5时,黏土矿物(110)表面的边络合物占优势,pH为8时,铀酰阳离子主要集中在黏土矿物(010)表面。利用先进的分子动力学模拟和光谱技术,首次在纳米尺度上揭示了黏土矿物表面吸附铀酰配合物的结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Uranium coordination on clay surface at nanoscale: Integration of EXAFS data and Ab initio molecular dynamics
Understanding the interactions between uranium and clay minerals at the molecular level is crucial for addressing environmental contamination and nuclear waste management. In this work we are focusing on the determination the molecular-level structure of uranium (VI) surface species on smectite using a combination of EXAFS spectroscopy and ab initio molecular dynamics simulations (AIMD). A set of samples was prepared with uranyl cation adsorbed on bentonite clay of three deposits at various pH and uranium concentrations. To elucidate the structure of the adsorbed species at nanoscale, AIMD simulations were carried out for uranyl coordination models at basal surface and on edge sites of clay mineral. Linear combination fitting of experimental EXAFS spectra with those calculated from AIMD trajectories allowed us to specify the fractions of each adsorbed complex configuration present at pH 3, 5 and 8. At low pH, the speciation of the adsorbed uranyl cation is mainly comprised of outer-sphere basal complex. When pH is increased to 5, edge complexes at (110) surface become predominant, and at pH 8 uranyl cation is mainly localized at (010) surface of clay mineral. With the implementation of advanced molecular dynamics simulations and spectroscopic techniques, the obtained results for the first time reveal the structure of the adsorbed uranyl complexes on clay mineral surfaces at nanoscale.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Clay Science
地学-矿物学
CiteScore
10.30
自引率
10.70%
发文量
289
审稿时长
39 days
期刊介绍:
Applied Clay Science aims to be an international journal attracting high quality scientific papers on clays and clay minerals, including research papers, reviews, and technical notes. The journal covers typical subjects of Fundamental and Applied Clay Science such as:
• Synthesis and purification
• Structural, crystallographic and mineralogical properties of clays and clay minerals
• Thermal properties of clays and clay minerals
• Physico-chemical properties including i) surface and interface properties; ii) thermodynamic properties; iii) mechanical properties
• Interaction with water, with polar and apolar molecules
• Colloidal properties and rheology
• Adsorption, Intercalation, Ionic exchange
• Genesis and deposits of clay minerals
• Geology and geochemistry of clays
• Modification of clays and clay minerals properties by thermal and physical treatments
• Modification by chemical treatments with organic and inorganic molecules(organoclays, pillared clays)
• Modification by biological microorganisms. etc...
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


