(+)-三烯醇K的全合成
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-05-02
DOI:10.1021/acs.joc.5c0038310.1021/acs.joc.5c00383
引用次数: 0
摘要
报道了第一个全合成的三烯醇K,一种新型的三霉素同系物,在其19元的内酰胺环内具有二烯部分。关键步骤包括构建三取代苯环的两个顺序钯催化交叉偶联反应和组装C6-C18片段的两种不同策略。Co2(CO)8催化羰基化环氧化物开环提供了二烯片段,而后期Stewart-Grubbs催化剂介导的二烯-烯闭环复分解(RCM)实现了高效的大环化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
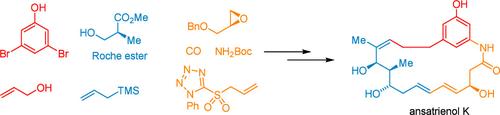
Total Synthesis of (+)-Ansatrienol K
The first total synthesis of ansatrienol K, a novel trienomycin congener featuring a diene moiety within its 19-membered macrolactam ring, is reported. Key steps include two sequential palladium-catalyzed cross-coupling reactions for constructing the trisubstituted benzene ring and two distinct strategies for assembling the C6–C18 fragment. A Co2(CO)8-catalyzed carbonylative epoxide ring-opening provided the diene fragment, while late-stage Stewart–Grubbs catalyst-mediated diene-ene ring-closing metathesis (RCM) enabled efficient macrocyclization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


