荧光4-硝基苯并-2-氧杂-1,3-二唑偶联胆汁酸作为溶质载体家族SLC10和SLCO肝脏和肠道胆汁酸转运体的探针底物
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
几种胆汁酸转运蛋白参与肝脏和肠道之间的肠肝胆汁酸循环,包括肝Na+/牛磺胆酸共转运多肽(NTCP)和肠尖钠依赖性胆汁酸转运蛋白(ASBT)。荧光BA衍生物有助于测量和可视化BA在体外和体内的转运。我们以4-硝基苯并-2-氧杂-1,3-二唑(NBD)作为标记荧光团,合成了一系列3-NBD偶联的BA。3α- nbd -牛磺胆酸、3β- nbd -牛磺胆酸、3α- nbd -糖胆酸和3β- nbd -糖胆酸对人NTCP、小鼠mNtcp和小鼠mAsbt具有显著的转运率,而人ASBT仅对3α- nbd -糖胆酸具有可靠的转运活性。总的来说,NBD与3α-位置的偶联优于3β-位置,与甘氨酸偶联的NBD- ba表现出最高的总转运率。合成的NBD-BA均未通过有机阴离子转运多肽OATP1B1和OATP1B3进行转运。总的来说,3α- nbd -胆酸最适合用于基于荧光的转运试验来评估NTCP和ASBT抑制剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
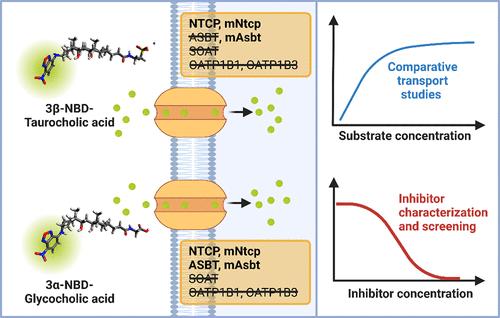
Fluorescent 4-Nitrobenzo-2-oxa-1,3-diazole-Coupled Bile Acids as Probe Substrates of Hepatic and Intestinal Bile Acid Transporters of the Solute Carrier Families SLC10 and SLCO
Several bile acid (BA) transporters are involved in the enterohepatic BA circulation between the liver and gut, including the hepatic Na+/taurocholate cotransporting polypeptide (NTCP) and the intestinal apical sodium-dependent BA transporter (ASBT). Fluorescent BA derivatives are helpful to measure and visualize BA transport in vitro and in vivo. We used 4-nitrobenzo-2-oxa-1,3-diazole (NBD) as the labeling fluorophore and synthesized a series of 3-NBD-coupled BA. While 3α-NBD-taurocholic acid, 3β-NBD-taurocholic acid, 3α-NBD-glycocholic acid, and 3β-NBD-glycocholic acid showed significant transport rates for human NTCP, mouse mNtcp, and mouse mAsbt, human ASBT only showed reliable transport activity for 3α-NBD-glycocholic acid. In general, NBD coupling to the 3α-position proved superior to the 3β-position, and the NBD-BA with glycine conjugation exhibited the highest overall transport rates. None of the synthesized NBD-BA was transported by the organic anion transporting polypeptides OATP1B1 and OATP1B3. Overall, 3α-NBD-glycocholic acid is most appropriate for fluorescence-based transport assays to evaluate NTCP and ASBT inhibitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


