基于实验和计算研究的白钨矿与方解石浮选分离的高效选择性抑制剂
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
白钨矿和方解石矿物含有相似的钙活性位点,导致表面成分溶解后相互转化。同时,传统的氧化矿石捕收剂缺乏选择性,难以将两种矿物浮选分离。研究了亚氨基二磺酸四钠在白钨矿和方解石浮选分离过程中的选择性吸附机理。通过单矿物和人工混合矿物试验,分析了两种矿物的浮选行为。结果表明,当TI浓度为5 × 10-4 mol/L时,白钨矿和方解石的回收率分别为88.69%和13.81%。说明TI选择性抑制方解石,实现了有效的浮选分离。TI在浆液中溶解,生成阴离子和钠离子。阴离子基团被吸附在矿物表面,使矿物表面的zeta电位发生负变化。这可能是由于TI的羧酸离子与方解石表面的Ca位点之间的配位反应,形成了co - Ca种。此外,TI的吸附增加了方解石表面特征离子COOH -的信号强度,形成具有簇状峰的致密吸附层。TI分子吸附在方解石(104)表面,形成亲水性膜,显著降低了表面疏水性。本研究有效地实现了白钨矿与含钙矿物的浮选分离,环保型有机抑制剂的研究与开发是今后研究的重点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
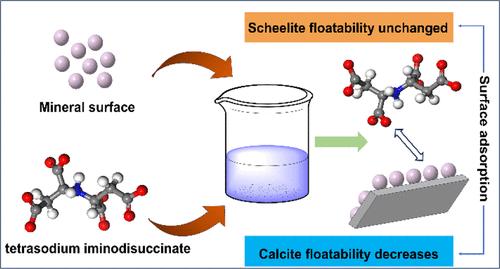
An Efficient Selective Depressant for the Flotation Separation of Scheelite from Calcite Based on Experimental and Calculational Studies
Scheelite and calcite minerals contain similar calcium-active sites, leading to a mutual transformation upon dissolution of surface components. At the same time, the traditional oxide ore collector lacks selectivity, and it is difficult to separate the two minerals by flotation. This study investigated the selective adsorption mechanism of tetrasodium iminodisuccinate (TI) during the flotation separation of scheelite and calcite. The flotation behavior of both minerals was analyzed through single-mineral and artificially mixed-mineral experiments. The results revealed that with the addition of a TI concentration of 5 × 10–4 mol/L as a depressant, scheelite and calcite exhibited recoveries of 88.69% and 13.81%, respectively. This indicates that TI selectively depressed calcite and achieved an effective flotation separation. TI undergoes dissolution in the slurry solution, generating anions and sodium ions. The anion groups are adsorbed on the mineral surface, negatively changing the zeta potential of the mineral surface. This was likely due to a coordination reaction between the carboxylate ions of TI and the Ca sites on the calcite surface, forming the COO–Ca species. Moreover, TI adsorption increased the signal intensity of the characteristic ion COOH– on the calcite surface, forming a dense adsorption layer with clustered peaks. TI molecules adsorbed onto the calcite (104) surface, creating a hydrophilic film that significantly reduced the surface hydrophobicity. This study effectively realizes the flotation separation of scheelite and calcium-containing minerals, and the research and development of an environmentally friendly organic depressant is the focus of future research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


