稳定MOF-808对水中四环素的有效去除:活化、稳定性及影响参数的综合研究
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
四环素是一种广泛使用的抗生素,引起了严重的环境问题。因此,实施有效的清除策略是减轻其环境影响的必要条件。研究了溶剂热法合成的MOF-808对TC的吸附性能。采用索氏提取和离心两种活化技术对合成的MOF-808进行了性能优化,得到的材料分别为S-MOF-808和C-MOF-808。对比研究表明,S-MOF-808的吸附性能优于C-MOF-808,因为它的BET表面积为1062 m2 g-1,而C-MOF-808的BET表面积为622 m2 g-1。两种MOF-808s的吸附实验均符合拟二级动力学模型和Langmuir等温模型。S-MOF-808对TC的最大吸附量约为333.33 mg g-1, C-MOF-808的最大吸附量约为312.50 mg g-1,表明S-MOF-808具有最佳的吸附性能。此外,经过两个月的化学稳定性评估,x射线衍射(XRD)分析表明,与C-MOF-808相比,S-MOF-808保持了更好的结构完整性。这些发现突出了S-MOF-808作为一种强大而有效的吸附剂从复杂的水环境中去除TC的潜力,并具有环境修复应用的适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
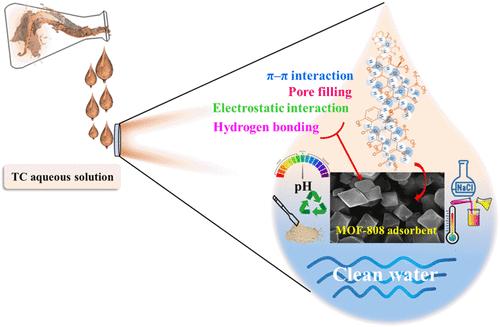
Effective Removal of Tetracycline from Water Using Stable MOF-808: A Comprehensive Investigation on Activation, Stability, and Influencing Parameters
Tetracycline (TC) is a widely utilized antibiotic that raises significant environmental concerns. Therefore, the implementation of effective removal strategies is imperative to mitigate its environmental impacts. This study investigates the adsorption of TC from aqueous solutions using MOF-808, synthesized via a solvothermal method. Two activation techniques, Soxhlet extraction and centrifugation, were applied to optimize the properties of the synthesized MOF-808, resulting in materials designated as S-MOF-808 and C-MOF-808, respectively. Comparative studies have demonstrated that S-MOF-808 shows superior adsorption due to its higher Brunauer–Emmett–Teller (BET) surface area of 1062 m2 g–1, compared to 622 m2 g–1 for C-MOF-808. The experimental adsorption results for both MOF-808s followed the pseudo-second-order kinetic and Langmuir isotherm models. The maximum adsorption capacities for TC were determined to be approximately 333.33 mg g–1 for S-MOF-808 and 312.50 mg g–1 for C-MOF-808, underscoring the optimal performance of S-MOF-808 in adsorption applications. Moreover, chemical stability was assessed over two months, with X-ray diffraction (XRD) analysis showing that S-MOF-808 maintained superior structural integrity compared to C-MOF-808. These findings highlight the potential of S-MOF-808 as a robust and efficient adsorbent for removing TC from complex aqueous environments, featuring its suitability for environmental remediation applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


