塑料/ k2feo4衍生多孔碳活化过氧单硫酸盐降解马拉硫磷制备低毒产物
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
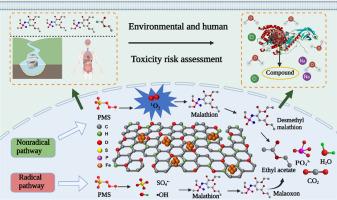
马拉硫磷是一种广泛使用的杀虫剂,对人体有毒性作用,被认为具有遗传毒性和致癌作用。•OH和SO4•−作为高级氧化过程中常见的自由基,可以有效降解马拉硫磷,但在此过程中容易产生剧毒产物马拉硫磷。针对这一问题,利用聚对苯二甲酸乙二醇酯(PET)塑料和K2FeO4制备了fe0掺杂的多孔塑料衍生碳材料(FAC)作为过氧单硫酸盐(PMS)触发器,对马拉硫磷进行了低毒降解。FAC (100 mg/L)可触发0.5 mM PMS在15 min内完全降解10 mg/L马拉硫磷。PMS单独、单线态氧(1O2)、羟基自由基(•OH)和硫酸盐自由基(SO4•−)对马拉硫磷的降解有促进作用,其中1O2的作用最大,贡献率为53.5%。利用密度泛函理论(DFT)确定了1O2与马拉硫磷的反应位置,进一步说明了1O2与去甲基马拉硫磷的反应。根据DFT程序,我们计算出了理论二阶速率常数,即1O2与马拉硫磷反应生成去甲基马拉硫磷的活性为1.88 × 1012 M−1 s−1,远高于另一条与高毒性产物马拉硫磷反应的反应途径(1.36 × 107 M−1 s−1)。用分子动力学方法分析了斑马鱼和人体各关键蛋白对降解产物的结合能,以表征其生态毒性和人体毒性。令人惊讶的是,与以往研究中毒性很高的中间体马拉硫磷相比,FAC/PMS体系中主要降解产物去甲基马拉硫磷的毒性却明显较低。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Degradation of malathion to low-toxicity products via peroxymonosulfate activated by plastic/K2FeO4-derived porous carbon
Malathion is a widely used insecticide with toxic effects on humans and is considered to be genotoxic and carcinogenic. As the common free radicals in advanced oxidation processes, hydroxyl radical (•OH) and sulfate radical (SO4•−) can efficiently degrade malathion, but highly toxic product malaoxon is prone to produce in this process. In response to this issue, polyethyleneterephthalate (PET) plastics and K2FeO4 were utilized to prepare Fe0-doped porous plastic-derived carbon material (FAC) as peroxymonosulfate (PMS) trigger to perform low toxicity degradation of malathion. FAC (100 mg/L) could trigger 0.5 mM PMS to completely degrade 10 mg/L malathion within 15 min. PMS alone, singlet oxygen (1O2), •OH, and SO4•− contributed to the degradation of malathion, in which 1O2 played the most important role with a contribution of 53.5%. Density functional theory (DFT) was employed to elucidate the reaction site of 1O2 for malathion, further illustrating 1O2 with the product of desmethyl malathion. Based on the DFT program, we calculated the theoretical second-order rate constants, the reactivity of 1O2 with malathion to produce desmethyl malathion, was 1.88 × 1012 M−1 s−1, which was much higher than another reaction pathway with the highly toxic product of malaoxon (1.36 × 107 M−1 s−1). The binding energies of various key proteins of zebrafish and human beings to the degradation products were analyzed by molecular dynamics to characterize their ecological and human toxicity. Surprisingly, in contrast to the highly toxic intermediate malaoxon in the previous studies, desmethyl malathion, the main degradation product in FAC/PMS system, has a significantly low toxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


