无定形LiNxHy在Co/MgO催化剂上促进低温氨分解
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
随着人们对氢能的兴趣日益浓厚,LiNH2和Li2NH等锂离子催化氨分解引起了越来越多的关注。然而,锂辅助催化剂的活性位点要求和反应机理仍存在争议。在这项研究中,我们证明了锂元素的加入显著提高了钴基催化剂在623 K时的氨分解率高达5倍,并且在报道的无钌催化剂中几乎达到了最好的低温活性。结构表征和密度泛函理论(DFT)计算表明,位于非晶LiNxHy和Co表面之间的界面上的空位Li位点是氨分解的活性位点。氨裂解过程中N-H键断裂步骤中,位于该空位位置的Li原子在与Co表面的直接弱相互作用下,每个Li原子的位移为1.7 Å,从而构建了有利于能量的几何结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
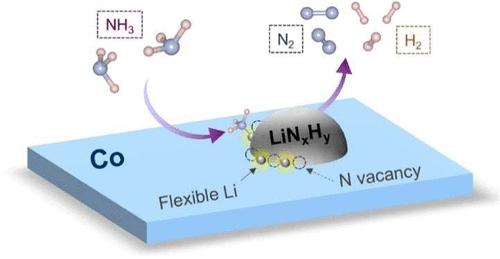
Amorphous LiNxHy Boosts Low-Temperature Ammonia Decomposition over the Co/MgO Catalyst
Catalytic ammonia decomposition facilitated by lithium species such as LiNH2 and Li2NH has attracted increasing attention alongside growing interest in hydrogen energy. However, the active site requirements and reaction mechanisms of Li-assisted catalysts remain controversial. In this study, we demonstrate that the incorporation of lithium species significantly enhances the ammonia decomposition rate of a cobalt-based catalyst by up to 5-fold at 623 K and achieves almost the best low-temperature activity among reported Ru-free catalysts. Structural characterization and density functional theory (DFT) calculations suggest that Li sites with vacancies, located at the interface between the amorphous LiNxHy species and the Co surface, serve as the active sites for ammonia decomposition. For the N–H bond scission step during ammonia cracking, Li atoms located at this vacancy site exhibit a displacement of 1.7 Å per Li atom under a direct weak interaction with the Co surface to construct the energy-favorable geometry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


