仿生纳米药物通过平衡调节肝细胞氧化应激和库普弗细胞炎症,提供柚皮苷增强急性肝衰竭治疗
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
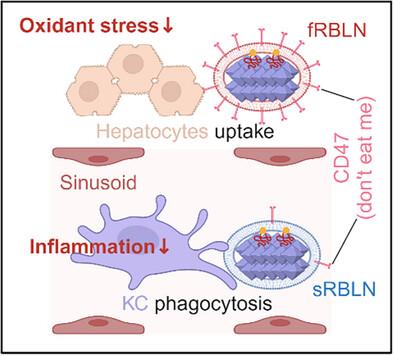
药物性急性肝衰竭(ALF)的特点是氧化应激和KC介导的炎症引起的肝细胞快速坏死,由于治疗窗口狭窄,治疗选择有限。为了应对这一挑战,一种仿生纳米药物(RBLN)被开发出来,用于平衡给药到肝细胞和肝细胞。层状双氢氧化物纳米颗粒(临床上用作Talcid)装载抗氧化剂柚皮苷,然后在新鲜(fRBLN)或衰老(sRBLN)状态下涂覆红细胞膜,利用KCs对衰老红细胞的优先清除。这种细胞膜涂层使fRBLN能够逃避KC的清除并靶向肝细胞,而sRBLN则被KC选择性地内化。静脉给药fRBLN和sRBLN的1:1组合以平衡的方式有效地将柚皮苷传递给肝细胞和KCs,降低肝细胞氧化应激,并通过将KCs从M1型极化到M2型来减轻kc驱动的炎症。该预处理可显著减轻药物性肝损伤,使模型小鼠肝功能基本恢复。该策略为平衡肝细胞靶向药物递送提供了一种新的范例,具有潜在的ALF治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Biomimetic Nanomedicines Deliver Naringin for Enhanced Acute Liver Failure Therapy via Balanced Regulation of Hepatocyte Oxidative Stress and Kupffer Cell Inflammation
Drug-induced acute liver failure (ALF) is characterized by rapid hepatocyte necrosis caused by oxidative stress and Kupffer cell (KC)-mediated inflammation, with limited therapeutic options due to narrow treatment windows. To target this challenge, a biomimetic nanomedicine (RBLN) is developed for balanced drug delivery to both hepatocytes and KCs. Layered double hydroxide nanoparticles (clinically used as Talcid) are loaded with the antioxidant naringin and then coated with red blood cell membranes in fresh (fRBLN) or senescent (sRBLN) states, leveraging the preferential clearance of senescent red blood cells by KCs. This cell membrane coating enabled fRBLN to evade KC clearance and target hepatocytes, while sRBLN is selectively internalized by KCs. Intravenous administration of a 1:1 combination of fRBLN and sRBLN efficiently delivered naringin to both hepatocytes and KCs in a balanced manner, reduced hepatocyte oxidative stress, and mitigated KC-driven inflammation by polarizing KCs from the M1 to M2 phenotype. Such a pre-treatment significantly alleviated drug-induced liver damage and nearly restored the liver functions in the mouse model. This strategy introduces a novel paradigm for balanced liver cell-targeted drug delivery with promising potential for ALF therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


