烯烃的有机催化区域选择性和立体选择性环丙烷化
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
作为天然产物和生物活性分子的活性中间体和亚结构,最小的环烷烃-环丙烷-是化学家们关注的一类分子。可以说,最普遍的化学合成方法是在烯烃中加入金属碳。虽然已有报道使用手性金属配合物和工程金属酶催化烯烃与类碳化合物的不对称环丙化反应,但我们现在报道了一种互补的、不含金属的、高度对映选择性的烯烃与重氮烷烃的环丙化反应,应用不对称反阴离子定向光氧化还原有机催化。我们确定了一个离子对,具有硫代蒽醌光氧化还原阳离子和一个手性酰亚胺二磷酰亚胺反阴离子,催化苯乙烯和脂肪二烯与重氮化合物的高度对映选择性环丙烷化。机理研究揭示了对映体选择性的波长依赖性,并表明主要的催化途径是通过烯烃衍生的自由基阳离子中间体进行的。这种无金属,高度对映选择性的有机催化方法补充了先前报道的烯烃操作方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
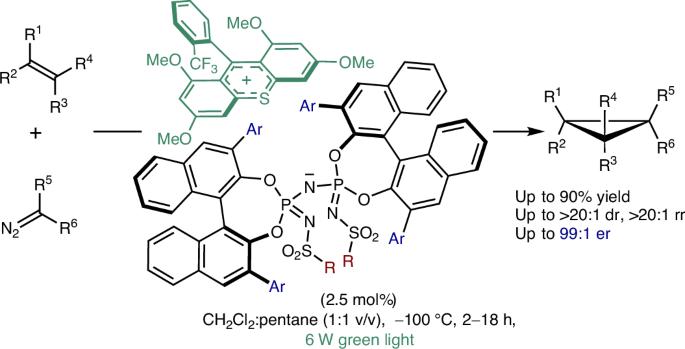

Organocatalytic regio- and stereoselective cyclopropanation of olefins
As reactive intermediates and substructures of natural products and bioactive molecules, the smallest cyclic alkanes—cyclopropanes—are an attractive class of molecules for chemists. Arguably, the most general approach to their chemical synthesis involves the addition of metal carbenes to olefins. Whereas catalytic asymmetric cyclopropanations of electronically unbiased olefins with carbenoids have been reported using chiral metal complexes and engineered metalloenzymes, we now report a complementary, metal-free and highly enantioselective cyclopropanation of olefins with diazoalkanes, applying asymmetric counteranion-directed photoredox organocatalysis. We identify an ion pair featuring a thioxanthylium photoredox cation and a chiral imidodiphosphorimidate counteranion that catalyses highly enantioselective cyclopropanations of styrenes and aliphatic dienes with diazo compounds. Mechanistic investigations reveal a wavelength dependence of the enantioselectivity and suggest that the main catalytic pathway proceeds via olefin-derived radical cation intermediates. This metal-free, highly enantioselective organocatalytic approach complements previously reported methods for alkene manipulations. Transformations from carbenes to olefins have generally been realized with transition metal-catalysed enantioselective methods or artificial metalloenzymes. Here the authors apply asymmetric counteranion-directed photoredox organocatalysis for the highly enantioselective cyclopropanation of styrenes and aliphatic dienes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


