血浆来源的中型细胞外囊泡作为前列腺癌主动监测决策的活检选择的潜力。
IF 4.6
2区 医学
Q2 MEDICINE, RESEARCH & EXPERIMENTAL
Nanomedicine : nanotechnology, biology, and medicine
Pub Date : 2025-05-11
DOI:10.1016/j.nano.2025.102828
引用次数: 0
摘要
诊断前列腺癌(PCa)和对患者进行风险分层仍然具有挑战性,因为基于psa的方法在主动监测(as)决策方面缺乏准确性。细胞外囊泡(EVs)是由所有类型的细胞释放的膜状纳米大小的囊泡,可能包含潜在的前列腺癌诊断过程中有趣的材料。本研究利用纳米流式细胞术和miRNA谱分析了24例PCa患者的中型血浆ev (mev)的表面标记物和miRNA谱。在AS和非AS患者中,PSMA+ EVs与PSMA+CD9+ EVs的比例存在显著差异。此外,miR-99a-5p、miR-125b-5p、miR-145-5p和miR-365a-3p水平在非as患者中较高。这些发现表明,血浆来源的PSMA+ mev起源于前列腺,可能作为前列腺癌进展的生物标志物。基于纳米流细胞技术的EV表面标记分析结合miRNA分析为PSA测量提供了一种新的、无创的替代方法。这种方法可以改善AS患者的风险分层和决策,可能导致更好的结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
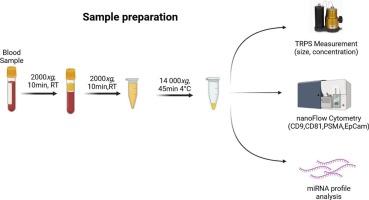
The potential of plasma-derived medium-sized extracellular vesicles as a biopsy alternative for active surveillance decisions in prostate Cancer
Diagnosing prostate cancer (PCa) and risk-stratifying patients remains challenging, as PSA-based methods lack precision for active surveillance (AS) decision-making. Extracellular vesicles (EVs) are membranous nano-sized vesicles released by all types of cells and may contain potentially interesting material for diagnostic procedures for PCa.
This study analyzed surface markers and miRNA profiles of medium-sized plasma EVs (mEVs) from 24 PCa patients using nanoflow cytometry and miRNA profiling. The ratio of PSMA+ EVs to PSMA+CD9+ EVs differed significantly between AS and non-AS patients. Additionally, miR-99a-5p, miR-125b-5p, miR-145-5p, and miR-365a-3p levels were higher in non-AS patients.
These findings suggest that plasma-derived PSMA+ mEVs originate from the prostate and may serve as biomarkers for PCa progression. Nanoflow cytometry-based analysis of EV surface markers combined with miRNA profiling provides a novel, non-invasive alternative to PSA measurements. This approach could improve risk stratification and decision-making for AS patients, potentially leading to better outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.10
自引率
0.00%
发文量
133
审稿时长
42 days
期刊介绍:
The mission of Nanomedicine: Nanotechnology, Biology, and Medicine (Nanomedicine: NBM) is to promote the emerging interdisciplinary field of nanomedicine.
Nanomedicine: NBM is an international, peer-reviewed journal presenting novel, significant, and interdisciplinary theoretical and experimental results related to nanoscience and nanotechnology in the life and health sciences. Content includes basic, translational, and clinical research addressing diagnosis, treatment, monitoring, prediction, and prevention of diseases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


