斑马鱼肠上皮损伤模型显示巨噬细胞和igfbp1a是粘膜愈合的主要调节剂。
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
促进肠道再生和增强粘膜愈合已成为治疗损害上皮屏障完整性和功能的肠道疾病的有希望的治疗选择。然而,这些过程背后的细胞和分子机制仍然知之甚少。这种知识差距部分是由于缺乏可靠和具有成本效益的体内模型来研究肠道损伤和再生的机制。在这里,我们建立了一个受控的、诱导的、靶向的肠上皮细胞(IEC)消融转基因斑马鱼模型,该模型再现了在人类中观察到的肠道损伤和再生的特征。单细胞RNAseq和实时成像显示巨噬细胞在恢复的肠道中积累,有助于其再生。此外,我们观察到胰岛素样生长因子结合蛋白1a (igfbp1a)在肠道损伤期间过表达。morpholino介导的igfbp1a敲低加重了肠道损伤和随后的再生受损。总之,我们介绍了一种新的肠道损伤斑马鱼模型,该模型能够在体内进行高通量筛选,以识别和验证粘膜愈合和肠道再生的新型调节剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
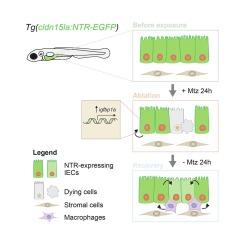
A zebrafish model of intestinal epithelial damage reveals macrophages and igfbp1a as major modulators of mucosal healing
Promoting intestinal regeneration and enhancing mucosal healing have emerged as promising therapeutic alternatives for treating intestinal disorders that compromise epithelial barrier integrity and function. However, the cellular and molecular mechanisms underlying these processes remain poorly understood. This knowledge gap is partly due to the lack of reliable and cost-effective in vivo models for studying the mechanisms governing intestinal damage and regeneration. Here, we developed a controlled, inducible, and targeted intestinal epithelial cell (IEC) ablation transgenic zebrafish model that recapitulates features of intestinal damage and regeneration observed in humans. Single-cell RNAseq and live imaging revealed accumulation of macrophages in the recovering intestine, contributing to its regeneration. Furthermore, we observed overexpression of insulin-like growth factor binding protein 1a (igfbp1a) during intestinal damage. Morpholino-mediated knockdown of igfbp1a exacerbated intestinal damage and impaired subsequent regeneration. In summary, we introduced a novel zebrafish model of intestinal damage that enables in vivo high-throughput screening for identifying and validating novel modulators of mucosal healing and intestinal regeneration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Mucosal Immunology
医学-免疫学
CiteScore
16.60
自引率
3.80%
发文量
100
审稿时长
12 days
期刊介绍:
Mucosal Immunology, the official publication of the Society of Mucosal Immunology (SMI), serves as a forum for both basic and clinical scientists to discuss immunity and inflammation involving mucosal tissues. It covers gastrointestinal, pulmonary, nasopharyngeal, oral, ocular, and genitourinary immunology through original research articles, scholarly reviews, commentaries, editorials, and letters. The journal gives equal consideration to basic, translational, and clinical studies and also serves as a primary communication channel for the SMI governing board and its members, featuring society news, meeting announcements, policy discussions, and job/training opportunities advertisements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


