手性1,3-二醇和氧烷对映互补合成环氧柠檬烯水解酶的研究
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
手性1,3-二醇和氧烷是合成关键药物的重要组成部分。在这项研究中,我们报道了柠檬烯环氧化物水解酶(LEHs)的鉴定和蛋白质工程,以实现外消旋氧烷的动力学分解,促进1,3-二醇的对映互补合成,同时获得光学纯净的氧烷。为了减少筛选工作,采用了单码和三码饱和诱变策略。底物范围扩大到包括12个例子,其中(R)和(S)选择性开环的外消旋氧烷的手性1,3-二醇产品和氧烷的光学纯度高达99%。结构和计算分析提供了对促进立体选择性的关键活性位点相互作用的见解。此外,通过配对卤代醇脱卤酶和LEHs变体,构建了双酶一锅两步级联反应,有效地将现成的外消旋卤代醇转化为手性1,3-二醇,分离收率高达48%,对映选择性高达98% ee。这些策略的可扩展性和实际适用性通过制备规模反应和随后的衍生化进一步证明,产生了关键的药物中间体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
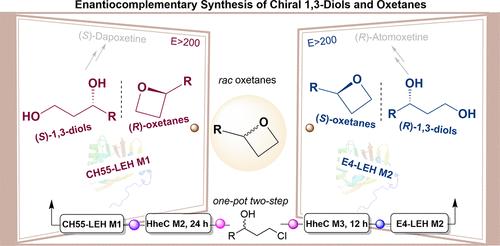
Engineering Limonene Epoxide Hydrolases for the Enantiocomplementary Synthesis of Chiral 1,3-Diols and Oxetanes
Chiral 1,3-diols and oxetanes serve as essential building blocks in the synthesis of key pharmaceuticals. In this study, we report the identification and protein engineering of limonene epoxide hydrolases (LEHs) to enable the kinetic resolution of racemic oxetanes, facilitating the enantiocomplementary synthesis of 1,3-diols while simultaneously obtaining optically pure oxetanes. To minimize screening efforts, single-code and triple-code saturation mutagenesis strategies were implemented. The substrate scope was expanded to include 12 examples where both (R)- and (S)-selective ring openings of racemic oxetanes were achieved in up to 99% optical purity for the chiral 1,3-diol products and oxetanes. Structural and computational analyses provided insights into key active-site interactions contributing to stereoselectivity. Furthermore, a bienzymatic one-pot two-step cascade reaction was constructed by pairing variants of halohydrin dehalogenase and LEHs, which efficiently converted readily available racemic haloalcohols into chiral 1,3-diols with high isolated yields (up to 48%) and enantioselectivity (up to 98% ee). The scalability and practical applicability of these strategies were further demonstrated through preparative-scale reactions and subsequent derivatizations, yielding key pharmaceutical intermediates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


