微塑料对菲在深度氧化过程中氧化降解的抑制作用:动力学和DFT研究
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
在这项研究中,我们研究了微塑料(MPs)在均相高级氧化过程(AOPs)中对菲(PHE)降解的抑制作用,包括Fenton、臭氧化和UV/H2O2过程。在没有MPs的情况下,所有三个AOPs的PHE都被完全去除。然而,MPs的存在降低了PHE的去除,这取决于MPs吸附的PHE的量。MPs负载的增加增加了对PHE去除的抑制作用,这是由于MPs表面对PHE的吸附增强;在AOPs过程中,PHE的氧化去除率随MPs吸附PHE的比例线性降低。这些抑制作用是由PHE吸附在PE-MPs上引起的,在很大程度上与水基质无关。动力学模型表明,聚乙烯(PE)-MPs与•OH吸附的PHE反应的二级速率常数(3.5 × 108 M-1s-1)比散装溶液中的PHE反应的二级速率常数(9.9 × 109 M-1s-1)低一个数量级以上。密度泛函理论计算表明,这种抑制作用是由于mps吸附的PHE和•OH之间的反应所需的活化能增加。PHE与•OH在PE-MPs表面反应的过渡态化学势估计比溶液反应的过渡态化学势高39% %。本文章由计算机程序翻译,如有差异,请以英文原文为准。
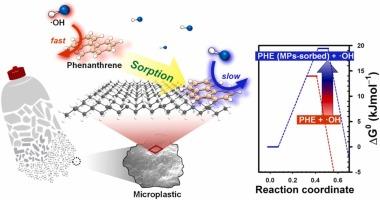
Inhibitory effects of microplastics on the oxidative degradation of phenanthrene during advanced oxidation processes: A kinetic and DFT study
In this study, we investigated the inhibitory effects of microplastics (MPs) on phenanthrene (PHE) degradation during homogeneous advanced oxidation processes (AOPs), including Fenton, ozonation, and UV/H2O2 processes. In the absence of MPs, PHE was completely removed in all three AOPs. However, the presence of MPs reduced the PHE removal, dependent on the amount of PHE adsorbed on MPs. An increase in MPs loading heightened the inhibitory effect on PHE removal due to enhanced adsorption of PHE onto the surface of MPs; the oxidative removal of PHE during AOPs decreased linearly with the fraction of PHE adsorbed onto MPs. These inhibitory effects, caused by PHE adsorption onto PE-MPs, were largely independent of the water matrix. Kinetic modeling revealed the second-order rate constant for the reaction of PHE adsorbed onto polyethylene(PE)-MPs with •OH (3.5 × 108 M–1s–1) to be more than an order of magnitude lower than that for PHE in bulk solution (9.9 × 109 M–1s–1). Density functional theory calculations indicated that this inhibitory effect arises from the increased activation energy required for the reaction between MPs-adsorbed PHE and •OH. The chemical potential of the transition state for the reaction of PHE with •OH on the PE-MPs surface was estimated to be 39 % higher than that for the reaction in solution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


