STAT5和STAT3的平衡影响树突状细胞功能和肿瘤免疫
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
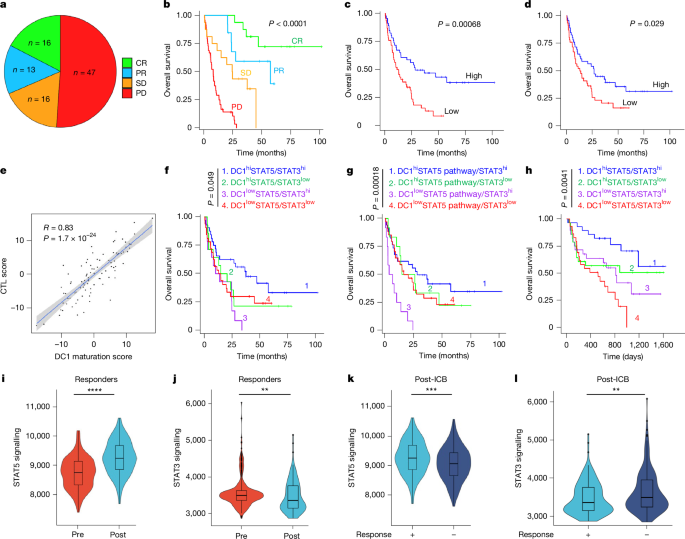
免疫检查点阻断(ICB)已经改变了癌症治疗1,2。免疫治疗的效果取决于树突状细胞介导的肿瘤抗原呈递、T细胞启动和激活3,4。然而,树突状细胞中的关键转录因子与ICB疗效之间的关系尚不清楚。在这里,我们发现ICB重编程树突状细胞中STAT3和STAT5转录途径之间的相互作用,从而激活T细胞免疫并使ICB有效。在机制上,STAT3抑制JAK2和STAT5的转录途径,决定树突状细胞功能的命运。由于STAT3经常在肿瘤微环境中被激活,我们开发了STAT3的两种不同的PROTAC(蛋白水解靶向嵌合体)降解物SD-36和SD-2301。STAT3降解物有效地降解树突状细胞中的STAT3,并重新编程树突状细胞转录网络,使其具有免疫原性。此外,STAT3降解剂单药治疗对晚期肿瘤和抗icb肿瘤有效,对小鼠无毒性。因此,STAT3和STAT5转录途径之间的串音决定了肿瘤微环境中的树突状细胞表型,STAT3降解物有望用于癌症免疫治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。

STAT5 and STAT3 balance shapes dendritic cell function and tumour immunity
Immune checkpoint blockade (ICB) has transformed cancer therapy1,2. The efficacy of immunotherapy depends on dendritic cell-mediated tumour antigen presentation, T cell priming and activation3,4. However, the relationship between the key transcription factors in dendritic cells and ICB efficacy remains unknown. Here we found that ICB reprograms the interplay between the STAT3 and STAT5 transcriptional pathways in dendritic cells, thereby activating T cell immunity and enabling ICB efficacy. Mechanistically, STAT3 restrained the JAK2 and STAT5 transcriptional pathway, determining the fate of dendritic cell function. As STAT3 is often activated in the tumour microenvironment5, we developed two distinct PROTAC (proteolysis-targeting chimera) degraders of STAT3, SD-36 and SD-2301. STAT3 degraders effectively degraded STAT3 in dendritic cells and reprogrammed the dendritic cell–transcriptional network towards immunogenicity. Furthermore, STAT3 degrader monotherapy was efficacious in treatment of advanced tumours and ICB-resistant tumours without toxicity in mice. Thus, the crosstalk between STAT3 and STAT5 transcriptional pathways determines the dendritic cell phenotype in the tumour microenvironment and STAT3 degraders hold promise for cancer immunotherapy. Immune checkpoint blockade activates T cell immunity by reprogramming the STAT3 and STAT5 transcriptional pathways in dendritic cells, and STAT3 degradation is effective for treating tumours of multiple cancer types in mouse models.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


