载脂蛋白L蛋白靶向共生体调节肠道免疫
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
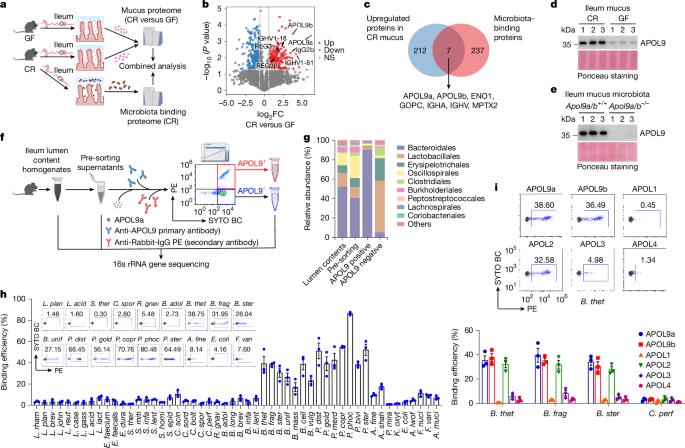
哺乳动物肠道内有数万亿的共生细菌,它们通过各种生物活性分子与宿主相互作用1,2。然而,宿主从这些共生关系中获益的互惠策略在很大程度上尚未被探索。在这里,我们报告了小鼠肠细胞在微生物群存在的情况下分泌载脂蛋白L9a和b (APOL9a/b)。通过将apol9结合细菌分类群的流式细胞术分选与16S核糖体RNA基因测序(APOL9-seq)相结合,我们发现APOL9a/b及其人类等效APOL2通过共生神经酰胺-1-磷酸(Cer1P)脂质具有高度特异性,覆盖了属于拟杆菌目的肠道细菌。肠道优势共生体拟杆菌(Bacteroides thetaiotaomicron)中神经酰胺-1-磷酸合成途径的遗传取消显著降低了APOL9a/b与细菌的结合。APOL9a/b包被诱导目标细菌产生外膜囊泡(OMVs),而不是裂解细菌细胞。随后,拟杆菌诱导的外膜囊泡增强宿主的干扰素-γ信号传导,促进肠上皮细胞中主要组织相容性复合体II类的表达。在小鼠中,Apol9a/b的缺失会损害肠道主要组织相容性复合体ii类指示的免疫屏障功能,导致肠道病原体感染的早期死亡。我们的数据显示了宿主诱导因子如何通过选择性靶向共生神经酰胺分子来促进肠道免疫稳态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeting symbionts by apolipoprotein L proteins modulates gut immunity
The mammalian gut harbours trillions of commensal bacteria that interact with their hosts through various bioactive molecules1,2. However, the mutualistic strategies that hosts evolve to benefit from these symbiotic relationships are largely unexplored. Here we report that mouse enterocytes secrete apolipoprotein L9a and b (APOL9a/b) in the presence of microbiota. By integrating flow cytometry sorting of APOL9-binding bacterial taxa with 16S ribosomal RNA gene sequencing (APOL9-seq), we identify that APOL9a/b, as well as their human equivalent APOL2, coat gut bacteria belonging to the order of Bacteroidales with a high degree of specificity through commensal ceramide-1-phosphate (Cer1P) lipids. Genetic abolition of ceramide-1-phosphate synthesis pathways in gut-dominant symbiote Bacteroides thetaiotaomicron significantly decreases the binding of APOL9a/b to the bacterium. Instead of lysing the bacterial cells, coating of APOL9a/b induces the production of outer membrane vesicles (OMVs) from the target bacteria. Subsequently, the Bacteroides-elicited outer membrane vesicles enhance the host’s interferon-γ signalling to promote major histocompatibility complex class II expression in the intestinal epithelial cells. In mice, the loss of Apol9a/b compromises the gut major histocompatibility complex class II-instructed immune barrier function, leading to early mortality from infection by intestinal pathogens. Our data show how a host-elicited factor benefits gut immunological homeostasis by selectively targeting commensal ceramide molecules. APOL9a/b proteins coat mouse intestinal bacteria with high specificity, and genetic abolition of ceramide-1-phosphate synthesis pathways in the symbiote Bacteroides thetaiotaomicron significantly decreases this binding of APOL9a/b to the bacterium.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


