质子感应介导的GPCR逐步激活的结构基础。
IF 25.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
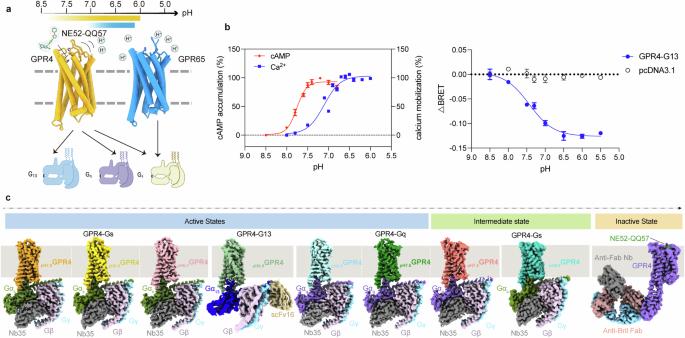
pH稳态的调节在许多生物过程中对生命的生存、生长和功能至关重要。包括GPR4、GPR65和GPR68在内的ph感应G蛋白偶联受体(gpcr)在检测细胞外质子浓度变化中发挥关键作用,影响生理和病理状态。然而,对质子感应机制的全面理解仍然是难以捉摸的。在这里,我们测定了GPR4和GPR65在不同pH水平下的不同激活状态下的低温电镜结构,结合Gs、Gq或G13蛋白,以及一个小分子ne52 - qq57结合的无活性GPR4结构。这些结构揭示了细胞外环2的动态性质及其在不同受体状态下的特征构象,揭示了细胞外组氨酸和羧酸残基网络介导的质子感应机制。值得注意的是,我们意外地捕获了GPR4- gs和GPR4- gq复合物的部分活性中间状态,并在GPR4中鉴定了NE52-QQ57的独特变构结合位点。通过将先前的研究与我们的结构分析和诱变数据相结合,我们提出了一个详细的质子感觉和GPCR激活的原子模型。这些见解可能为开发选择性配体和针对pH传感相关疾病的靶向治疗干预铺平道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structural basis of stepwise proton sensing-mediated GPCR activation
The regulation of pH homeostasis is crucial in many biological processes vital for survival, growth, and function of life. The pH-sensing G protein-coupled receptors (GPCRs), including GPR4, GPR65 and GPR68, play a pivotal role in detecting changes in extracellular proton concentrations, impacting both physiological and pathological states. However, comprehensive understanding of the proton sensing mechanism is still elusive. Here, we determined the cryo-electron microscopy structures of GPR4 and GPR65 in various activation states across different pH levels, coupled with Gs, Gq or G13 proteins, as well as a small molecule NE52-QQ57-bound inactive GPR4 structure. These structures reveal the dynamic nature of the extracellular loop 2 and its signature conformations in different receptor states, and disclose the proton sensing mechanism mediated by networks of extracellular histidine and carboxylic acid residues. Notably, we unexpectedly captured partially active intermediate states of both GPR4–Gs and GPR4–Gq complexes, and identified a unique allosteric binding site for NE52-QQ57 in GPR4. By integrating prior investigations with our structural analysis and mutagenesis data, we propose a detailed atomic model for stepwise proton sensation and GPCR activation. These insights may pave the way for the development of selective ligands and targeted therapeutic interventions for pH sensing-relevant diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


