cu - bpin介导的4,4-二氯-2-丁烯酸衍生物二聚化反应可以合成密集功能化的环丙烷。
IF 2.1
4区 化学
Q2 CHEMISTRY, ORGANIC
Beilstein Journal of Organic Chemistry
Pub Date : 2025-05-05
eCollection Date: 2025-01-01
DOI:10.3762/bjoc.21.71
引用次数: 0
摘要
4,4-二氯-2-丁酸衍生物在铜催化下与双(pinacolato)二硼反应时发生了罕见的二聚化过程。该反应提供了高度功能化的产物,具有优异的化学选择性、区域选择性和非对映选择性。这种高度的功能化使这些产品成为立体选择性合成氯环丙烷的通用构件。本文章由计算机程序翻译,如有差异,请以英文原文为准。
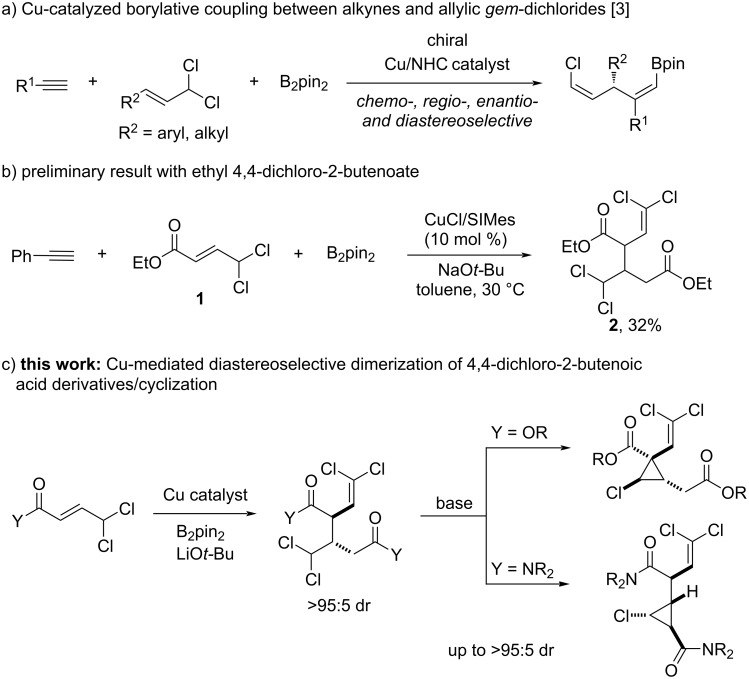
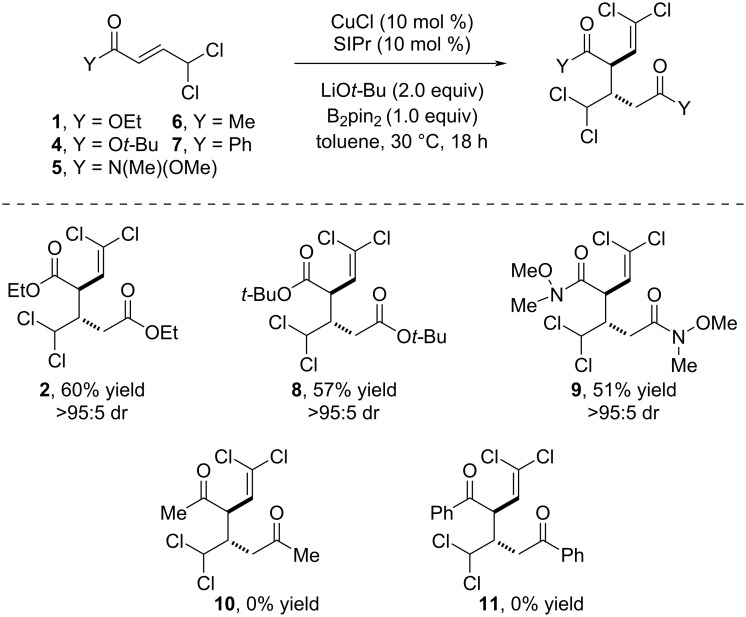
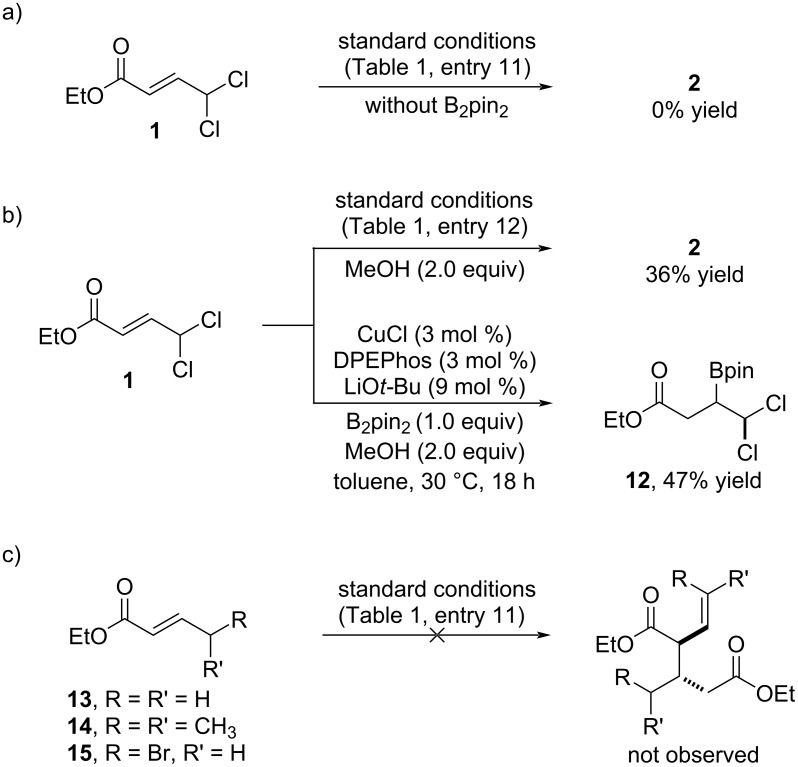
Cu-Bpin-mediated dimerization of 4,4-dichloro-2-butenoic acid derivatives enables the synthesis of densely functionalized cyclopropanes.
4,4-Dichloro-2-butenoic acid derivatives are shown to undergo a rare dimerization process when reacted with bis(pinacolato)diboron under copper catalysis. The reaction provides densely functionalized products with excellent levels of chemo-, regio-, and diastereoselectivity. This high degree of functionalization makes these products versatile building blocks for the stereoselective synthesis of chlorocyclopropanes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
4.90
自引率
3.70%
发文量
167
审稿时长
1.4 months
期刊介绍:
The Beilstein Journal of Organic Chemistry is an international, peer-reviewed, Open Access journal. It provides a unique platform for rapid publication without any charges (free for author and reader) – Platinum Open Access. The content is freely accessible 365 days a year to any user worldwide. Articles are available online immediately upon publication and are publicly archived in all major repositories. In addition, it provides a platform for publishing thematic issues (theme-based collections of articles) on topical issues in organic chemistry.
The journal publishes high quality research and reviews in all areas of organic chemistry, including organic synthesis, organic reactions, natural product chemistry, structural investigations, supramolecular chemistry and chemical biology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


