邻氨基酚的受底物控制的区域选择性C(sp2)-H磺化反应。
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
描述了Cu(II)催化邻氨基酚与磺酰肼的高区域选择性C(sp2)-H键磺化反应,得到芳基砜。磺酰肼通过单电子转移(SET)生成的磺酰自由基通过Cu(II)/Cu(III)催化循环与氨基苯酚形成C-S键。研究了合成的芳基砜的合成转化和光物理性质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
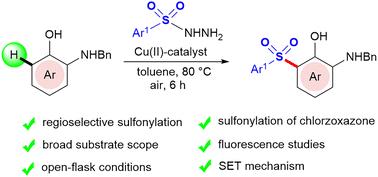
Substrate-controlled regioselective C(sp2)–H sulfonylation of ortho-aminophenols†
Cu(II)-catalyzed highly regioselective C(sp2)–H bond sulfonylation of ortho-aminophenols with sulfonyl hydrazides to obtain arylsulfones is described. The sulfonyl radical generated from sulfonyl hydrazide via single-electron transfer (SET) forms a C–S bond with the aminophenol via a Cu(II)/Cu(III) catalytic cycle. The synthetic transformations and photophysical properties of the synthesized aryl sulfones have also been investigated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


