析氧反应中高稳定活性镍电极的铁种再生。
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
为了实现碱水电解绿色制氢,提高镍电催化剂的析氧反应(OER)活性,同时保持高稳定性至关重要。在这里,我们提出了一个新的和有效的策略来实现这一目标,通过引入短的,周期性的再生步骤,辅之以精确调整电解质中铁的痕量。该策略允许在工业相关电流密度为300 mA cm-2、含铁量为1.0 M KOH的电解质(模拟商业电解质)下,在整个测试持续时间(72小时)内保持吸附在镍电极上的铁所带来的增强活性。在相同的条件下,但没有再生,大约18小时后观察到剧烈的失活。飞行时间二次离子质谱(ToF-SIMS)强调,这种失活与电极表面铁的损失有关。再生步骤有助于保留镍电极表面的铁,从而获得所需的高OER活性和稳定性。我们估计,与目前碱性电解槽的标准操作条件相比,这种再生策略可以节省高达18%的能源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
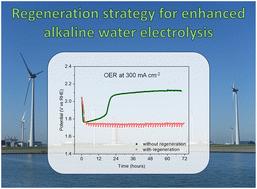
Regeneration of iron species for high and stable activity of nickel electrodes in the oxygen evolution reaction†
To enable green hydrogen production through alkaline water electrolysis, it is crucial to enhance the activity of nickel electrocatalysts towards the oxygen evolution reaction (OER), while preserving high stability. Here, we present a new and effective strategy to achieve this target through the introduction of short, periodical regeneration steps, complemented with the accurate tuning of traces of iron in the electrolyte. This strategy allowed retaining the enhanced activity brought about by the iron species adsorbed on the nickel electrode for the whole test duration (72 h) at an industrially relevant current density of 300 mA cm−2 with a 1.0 M KOH electrolyte containing ca. 100 ppb of iron (mimicking a commercial electrolyte). Under the same conditions but without regeneration, a dramatic deactivation was observed after ca. 18 h. Time-of-flight secondary ion mass spectrometry (ToF-SIMS) highlighted that such deactivation is correlated to the loss of iron species from the surface of the electrode. The regeneration steps help retain the iron species on the surface of the nickel electrode, thus granting the desired high OER activity and stability. We estimated that this regeneration strategy could lead to up to 18% energy saving compared to the current standard operating conditions of alkaline electrolysers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


