CREG1通过线粒体自噬促进骨髓间充质干细胞成骨分化,减轻骨质疏松症骨质流失
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
骨质疏松症的病理过程涉及骨吸收加速和骨形成减少,其中骨髓间充质干细胞(BMSCs)成脂分化和成骨分化平衡的破坏是至关重要的一部分。e1a刺激基因1的细胞抑制因子(CREG1)是一种小糖蛋白,主要定位于内核溶酶体腔室,与线粒体自噬和细胞分化的调节有关。然而,其在骨髓间充质干细胞成骨分化和骨骼退行性疾病(包括骨质疏松症)中的作用尚不清楚。我们之前通过数据库分析发现CREG1在骨髓中高表达,并发现其表达在骨髓间充质干细胞成骨分化过程中增加。在本研究中,我们证明了CREG1在骨质疏松症患者和动物模型中的表达降低,与卵巢切除术(OVX)诱导的骨质流失模型相比,CREG1的过表达导致了更高的骨量。进一步研究发现,CREG1的下调抑制了骨髓间充质干细胞的成骨分化,而CREG1的过表达则促进了这一过程。此外,我们发现CREG1过表达伴随着线粒体自噬水平的增加,当线粒体自噬被抑制时,这种过表达诱导的成骨分化被阻断,表明CREG1通过诱导线粒体自噬促进成骨分化。因此,我们的研究结果表明,CREG1参与调节骨髓间充质干细胞的成骨分化,从而为骨质疏松症的治疗提供新的治疗靶点和途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
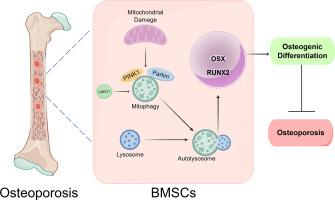
CREG1 alleviates bone loss in osteoporosis by enhancing the osteogenic differentiation of BMSCs through mitophagy
The pathological process of osteoporosis involves accelerated bone resorption and a decline in bone formation, among which the disruption of the balance between adipogenic and osteogenic differentiation in bone marrow mesenchymal stem cells (BMSCs) is a crucial part. Cellular repressor of E1A-stimulated genes 1 (CREG1), a small glycoprotein, is mainly localized to the endosomal-lysosomal compartment and is associated with the regulation of mitophagy and cell differentiation. However, its roles in BMSCs osteogenic differentiation and skeletal degenerative disorders, including osteoporosis, are poorly understood. We previously identified CREG1 as being highly expressed in the bone marrow through database analysis and found that its expression increased in the process of BMSCs osteogenic differentiation. In the present study, we demonstrated that the expression of CREG1 was reduced in osteoporosis patients and animal models, and the overexpression of CREG1 contributed to higher bone mass compared with ovariectomy (OVX)-induced bone loss models. Further research revealed that the knockdown of CREG1 inhibited the osteogenic differentiation of BMSCs, while CREG1 overexpression promoted this process. Additionally, we found that CREG1 overexpression was accompanied by an increase in mitophagy levels, and the osteogenic differentiation induced by this overexpression was blocked when mitophagy was inhibited, indicating that CREG1 promoted osteogenic differentiation through inducing mitophagy. Therefore, our findings demonstrated that CREG1 is involved in regulating the osteogenic differentiation of BMSCs, thereby providing new therapeutic targets and pathways for the treatment of osteoporosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


