靶向xpo1的选择性转录激活抑制剂抑制移植物抗宿主病
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
输出蛋白-1 (XPO1)是核到细胞质蛋白运输的中介。靶向xpo1的选择性核输出抑制剂(SINE) Selinexor被fda批准用于复发性血液恶性肿瘤。最近,我们报道了一类独特的XPO1调节剂,它们可以抑制T细胞激活而不损害核输出或细胞活力(选择性转录激活抑制剂,或SITAs),这表明XPO1可能是T细胞驱动疾病的治疗靶点。在此,我们分析了两个结构不同的SITAs亚序列的构效关系。一组含吡啶的结构获得了高细胞效价(EC50 1 nM),而一组互补的含吡咯三嗪的分子在细胞效价和理想的物理化学性质之间取得了平衡。这两个亚系列的铅分子在体内表现出XPO1的作用,在小鼠移植物抗宿主病模型中有效,并且对Selinexor表现出优异的耐受性。这项研究提供了证据,证明优化的靶向XPO1的sita可以将XPO1作为治疗靶点扩展到肿瘤以外的适应症。本文章由计算机程序翻译,如有差异,请以英文原文为准。
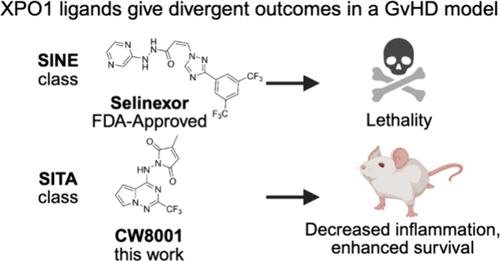
XPO1-Targeting Selective Inhibitors of Transcriptional Activation Suppress Graft-versus-Host Disease
Exportin-1 (XPO1) is a mediator of nuclear-to-cytoplasmic protein trafficking. The XPO1-targeting selective inhibitor of nuclear export (SINE) Selinexor is FDA-approved for relapsed hematological malignancies. Recently, we reported a unique class of XPO1 modulators that suppressed T cell activation without impairing nuclear export or cell viability (the selective inhibitors of transcriptional activation, or SITAs), suggesting that XPO1 may be a therapeutic target in T cell-driven diseases. Here, we analyzed structure–activity relationships of two structurally distinct subseries of SITAs. A set of pyridine-containing structures attained high cellular potencies (EC50 1 nM), while a complementary set of pyrrolotriazine-containing molecules balanced cellular potency with desirable physicochemical properties. Lead molecules from both subseries demonstrated in vivo XPO1 engagement, were efficacious in a mouse model of graft versus host disease, and showed superior tolerability to Selinexor. This study provides evidence that optimized XPO1-targeting SITAs can extend XPO1 as a therapeutic target to indications beyond oncology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


