Mg2+激活的DPSCs中富集mir -451a的细胞外小泡通过AKT/eNOS/NO轴诱导血管化骨再生
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
血管的形成是骨组织再生的必要部分。msc - sev在体内骨再生策略中起着至关重要的作用。然而,天然mscs - sev的血管形成能力有限,这使得其难以诱导血管化骨再生。本研究纯化了镁离子激活的DPSCs (Mg2+- ev)衍生的sev,发现其在促进内皮细胞迁移和血管生成、BMSC增殖和成骨方面具有卓越的潜力。Mg2+- ev的有益作用可能归因于miR-451a的富集以及随后对AKT/eNOS信号通路的调节和激活。在此基础上,Mg2+- ev在β- tcp修饰的GelMA支架上递送,具有缓释和更好的生物利用度。大鼠颅骨缺损模型验证了GelMA/β-TCP与Mg2+- ev具有增强诱导血管化骨再生的潜力。本研究提供了一种阳离子激活策略来调节msc衍生的sev的货物和含量,获得理想的血管促进和骨再生潜力。此外,开发的β- tcp修饰的递送支架代表了有效装载和缓释递送用于临床翻译的sev的有前途的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
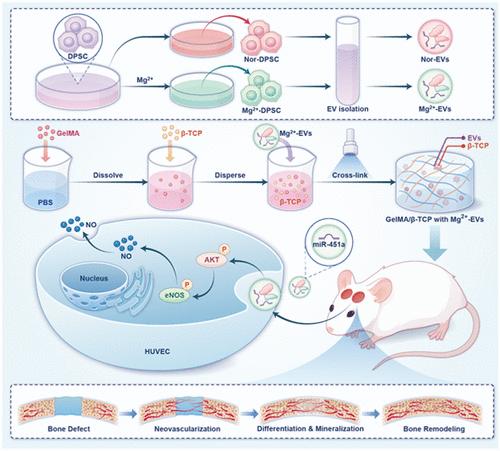
MiR-451a-Enriched Small Extracellular Vesicles Derived from Mg2+-Activated DPSCs Induce Vascularized Bone Regeneration through the AKT/eNOS/NO Axis
Blood vessel formation is a necessary part of bone tissue regeneration. MSCs-sEVs play a vital role in the in vivo bone regeneration strategy. However, natural MSCs-sEVs suffer from limited blood vessel formation potency, which makes it difficult to induce vascularized bone regeneration. Here, sEVs derived from magnesium cation-activated DPSCs (Mg2+-EVs) are purified and found to have superior potential in promoting endothelial cell migration and angiogenesis, as well as BMSC proliferation and osteogenesis. The beneficial effects of Mg2+-EVs could be attributed to the enrichment of miR-451a and the subsequent regulation and activation of AKT/eNOS signaling pathways. On this basis, Mg2+-EVs are delivered on β-TCP-modified GelMA scaffolds for slow release and better bioavailability. The rat cranial defect model verifies that GelMA/β-TCP with Mg2+-EVs has enhanced potential of inducing vascularized bone regeneration. The present study provides a cation-activated strategy to modulate the cargos and contents of MSC-derived sEVs, obtaining desirable vascular promotion and bone regeneration potential. Furthermore, the developed β-TCP-modified delivery scaffolds represent a promising strategy for efficient loading and slow-release delivery of sEVs for clinical translation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


