线粒体特异性GPX4抑制增强铁下垂和抗肿瘤免疫
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
作为一种潜在的癌症免疫治疗策略,铁凋亡正受到人们的关注,因为它可以通过增强树突状细胞(DC)的激活和细胞毒性T细胞(ctl)的浸润来刺激抗肿瘤反应。然而,癌细胞经常对铁下垂产生耐药性,降低了现有治疗的有效性。这项研究展示了一种新的线粒体靶向铁下垂诱导剂,命名为mitoFePDA@R,它被设计用于实现铁下垂诱导、抗肿瘤免疫激活和增强铁下垂的“闭环”癌症免疫治疗策略。在该策略中,mitoFePDA@R旨在释放肿瘤线粒体内的Fe2+和mitoGPX4抑制剂RSL3,从而有效诱导铁下垂并激活强大的抗肿瘤免疫反应。此外,ctl释放的干扰素γ (IFN-γ)抑制GSH合成,进一步增强肿瘤细胞对铁下垂的敏感性,形成“闭环”策略。体外研究表明mitoFePDA@R通过在线粒体中积累脂质过氧化物(LPO)(缺乏线粒体靶向)诱导肿瘤细胞强烈的铁凋亡。动物研究证实mitoFePDA@R有效触发铁下垂并激活随后的抗肿瘤免疫反应,导致显著的肿瘤生长抑制。这为铁中毒相关癌症的免疫治疗提供了一种可行和有效的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
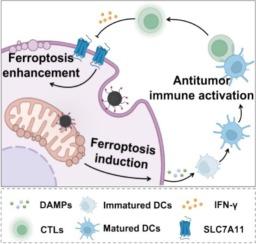
Mitochondria-specific GPX4 inhibition enhances ferroptosis and antitumor immunity
Ferroptosis is gaining attention as a potential cancer immunotherapy strategy, as it can stimulate antitumor responses by enhancing dendritic cell (DC) activation and the infiltration of cytotoxic T cells (CTLs). However, cancer cells often develop resistance to ferroptosis, reducing the effectiveness of existing treatments. This study demonstrates a novel mitochondria-targeted ferroptosis inducer, designated mitoFePDA@R, which is engineered to achieve a “closed-loop” cancer immunotherapy strategy of ferroptosis induction, antitumor immune activation, and ferroptosis enhancement. In this strategy, mitoFePDA@R is designed to release Fe2+ and the mitoGPX4 inhibitor RSL3 within tumor mitochondria, thereby effectively inducing ferroptosis and activating strong antitumor immune responses. Additionally, interferon γ (IFN-γ) released from CTLs inhibits GSH synthesis, which further enhances the ferroptosis sensitivity of tumor cells to form a “closed-loop” strategy. In vitro studies indicated that mitoFePDA@R induced strong ferroptosis in tumor cells by accumulating lipid peroxides (LPO) in mitochondria (which lacks mitochondria targeting). Animal studies confirmed that mitoFePDA@R effectively triggered ferroptosis and activated subsequent antitumor immune responses, leading to significant tumor growth inhibition. This provides a viable and effective strategy for ferroptosis-associated cancer immunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


