了解在温和pH溶液中水还原和水氧化的两个伏安特性
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
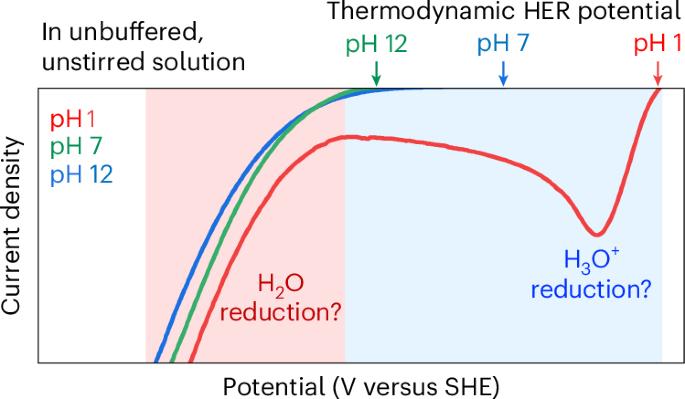
许多过程的电气化需要在温和的pH条件下使用水溶液,在这种条件下析氢反应(HER)和析氧反应(OER)可能成为竞争反应。在温和的pH条件下,HER和OER表现出特殊的伏安行为,特别是在强酸性和碱性溶液中没有观察到的两种还原或氧化特征。这些行为不能完全由热力学因素来解释,并且由于涉及多种水种(h30 +, H2O和OH -)以及这些水种之间通过水的自解离和酸碱中和反应的转化而特别复杂。本分析对pH、电位、搅拌和缓冲液对HER和OER的热力学和动力学的影响进行了系统和概念性的解释,为理解温和pH条件下HER和OER的行为及其对其他更广泛的水反应的影响提供了基础和必要的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Understanding two voltammetric features of water reduction and water oxidation in mild pH solutions
Electrification of many processes requires the use of aqueous solutions under mild pH conditions where the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER) can become competing reactions. The HER and OER under mild pH conditions show peculiar voltammetric behaviours, specifically two reductive or oxidative features, that are not observed in strongly acidic and basic solutions. These behaviours cannot be fully explained by thermodynamic considerations only and are particularly complex owing to the involvement of multiple water species (H3O+, H2O and OH–) and the conversion between these species via water autodissociation and acid–base neutralization reactions. This Analysis provides a systematic and conceptual explanation of the effect of pH, potential, stirring and buffer on the thermodynamics and kinetics of the HER and OER, providing fundamental and yet essential insights into comprehending HER and OER behaviours under mild pH conditions, and their implications for other aqueous reactions more broadly. The electrochemical behaviour observed during water reduction and oxidation is considerably more complex under mild pH than under strongly acidic or alkaline conditions. This Analysis explains the origins of this behaviour and presents its implications for aqueous electrocatalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


