光催化用自还原CaFe1-xCuxO3-δ钙钛矿:Cu掺杂与O-H基团的协同作用
IF 4.6
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
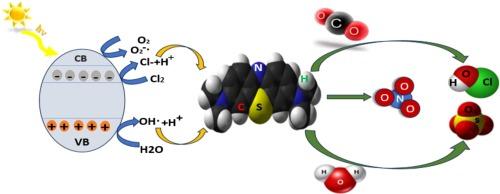
采用柠檬酸辅助溶胶-凝胶法制备了纯CaFeO3-δ钙钛矿纳米粉体和掺杂cu (1 wt%、4 wt%和10 wt%)的钙钛矿纳米粉体。为了保证羟基(O-H)的保存,前驱体在合成和干燥过程中用半透膜密封。随后在900°C下煅烧6 h,得到结晶良好的CaFeO3-δ相,主要为正构相,少量残余的Fe2−δHδO3和富含羟基的Ca(OH)2相。合成的材料在可见光照射下对亚甲基蓝(MB)具有良好的光催化降解活性。这种增强的性能归因于铜掺杂和羟基网络之间的协同相互作用,羟基网络作为类似电解质的介质促进电荷传输。值得注意的是,1 wt%的Cu掺杂表现出最佳的效率,在80分钟内实现91.21%的MB降解。Cu掺杂产生的载流子,加上羟基电解质实现的高效电荷分离,是显著的催化活性的原因。值得注意的是,cu掺杂的CaFeO3-δ钙钛矿作为一种自维持催化剂,利用其固有的羟基网络高效稳定地降解MB。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Self-reducing CaFe1-xCuxO3-δ perovskite for photocatalysis: The synergistic role of Cu doping and O–H groups
Pure and Cu-doped (1 wt%, 4 wt%, and 10 wt%) CaFeO3-δ perovskite nanopowders were synthesized via a citric acid-assisted sol–gel method. In order to ensure the preservation of hydroxyl (O–H) groups, the precursors were hermetically sealed with semipermeable Parafilm during synthesis and drying. Subsequent calcination at 900 °C for 6 h yielded a well-crystallized, predominantly orthorhombic CaFeO3-δ phase, along with minor residual Fe2−δHδO3 and Ca(OH)2 phases rich in hydroxyl groups. The synthesized materials exhibited exceptional photocatalytic activity for methylene blue (MB) degradation under visible-light irradiation. This enhanced performance is attributed to the synergistic interplay between copper doping and the hydroxyl network, which acts as an electrolyte-like medium to facilitate charge transport. It is noteworthy that 1 wt% Cu-doping demonstrated optimal efficiency, achieving 91.21 % MB degradation within 80 min. The charge carriers generated by Cu doping, coupled with efficient charge separation enabled by the hydroxyl electrolyte, are responsible for the remarkable catalytic activity. Notably, the Cu-doped CaFeO3-δ perovskite functions as a self-sustaining catalyst, utilizing its intrinsic hydroxyl network to degrade MB with high efficiency and stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Science and Engineering: B
工程技术-材料科学:综合
CiteScore
5.60
自引率
2.80%
发文量
481
审稿时长
3.5 months
期刊介绍:
The journal provides an international medium for the publication of theoretical and experimental studies and reviews related to the electronic, electrochemical, ionic, magnetic, optical, and biosensing properties of solid state materials in bulk, thin film and particulate forms. Papers dealing with synthesis, processing, characterization, structure, physical properties and computational aspects of nano-crystalline, crystalline, amorphous and glassy forms of ceramics, semiconductors, layered insertion compounds, low-dimensional compounds and systems, fast-ion conductors, polymers and dielectrics are viewed as suitable for publication. Articles focused on nano-structured aspects of these advanced solid-state materials will also be considered suitable.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


