铜上CO2电还原过程中温度对表面CO族的影响
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在工业应用中,由于传热限制,CO2电解槽可能在高温下运行,但温度对CO2电还原中涉及的表面反应的影响仍然难以捉摸,并且在很大程度上基于产品分析的推断。在这项研究中,我们使用表面增强红外吸收光谱(SEIRAS)来反卷积温度依赖性现象,影响铜在20至80°C之间的CO族。我们发现,CO的覆盖和向缺陷位点的迁移在20 - 45°C之间增加,在45 - 80°C之间减少,这表明温度的升高有利于CO加氢生成C1产物,而不是CO偶联生成C2+产物。C1和C2+产物的生成速率分别与缺陷位点上CO的浓度有1.28和1.95阶依赖关系,表明缺陷位点在20 ~ 80°C之间是产物生成的活性位点。因此,温度升高对CO转化为C1和C2+产物的途径有直接影响,而不仅仅是控制局部CO2的可用性、质量传递和基本反应速率。这些发现提供了对高温下潜在反应机制的更深入理解,这是使产品分配合理化和设计提高二氧化碳电解槽中C2+产量的解决方案的关键一步。本文章由计算机程序翻译,如有差异,请以英文原文为准。
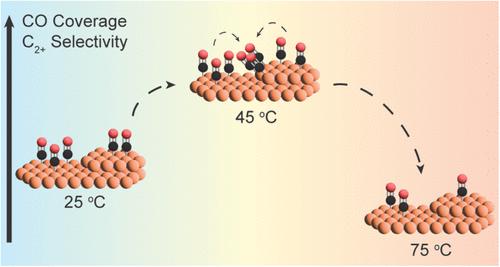
Temperature Effects on the Surface CO Population during CO2 Electroreduction over Copper
In industrial implementations, CO2 electrolyzers will likely operate at high temperatures due to heat transfer limitations, but the effects of temperature on surface reactions involved in CO2 electroreduction remain elusive and heavily based on inference from product analysis. In this study, we used surface-enhanced infrared absorption spectroscopy (SEIRAS) to deconvolute temperature-dependent phenomena affecting the CO population on copper between 20 and 80 °C. We show that CO coverage and migration to defect sites increase between 20 and 45 °C and decrease between 45 and 80 °C, suggesting that increasing temperature favors a CO hydrogenation route to C1 products over a CO coupling route to C2+ products. C1 and C2+ product formation rates have 1.28 and 1.95 order dependence on the concentration of CO on defect sites, respectively, indicating that these are the active sites for product formation between 20 and 80 °C. Thus, increasing temperature has a direct effect on the CO conversion route to C1 and C2+ products beyond just controlling local CO2 availability, mass transport, and elementary reaction rates. These findings provide a deeper understanding of the underlying reaction mechanism at elevated temperatures, which is a key step in rationalizing product distribution and in designing solutions for enhanced C2+ production in CO2 electrolyzers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


