no2促进棕色碳上SO2的非均相光化学氧化生成硫酸盐
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
NO2在典型褐碳(BrC)物种(荧光蒽,FL)吸收SO2形成硫酸盐的非均相光化学过程中的作用;4-(苯甲酰)苯甲酸,4- bba;采用红外光谱法、离子色谱法和理论计算对3,7-二羟基-2-萘酸(3,7-脱氧麻酸)进行了系统的探索。NO2增强了BrC对SO2的吸收和硫酸盐的生成,表现为SO2的稳态吸收系数和硫酸盐形成速率的增大。BrC上光电子对NO2的还原可以产生NO2−,并被证明是促进SO2氧化成硫酸盐的触发因素。在FL上最容易形成NO2 -,其次是4 - BBA和3,7 - dna,这可以通过生成NO2 -的能量势垒来证明:FL (0.01 kJ mol-1) <;4−BBA (0.12 kJ mol-1) <;3,7−脱氧核糖核酸(1.33 kJ mol-1)。SO2/NO2与BrC反应的SO2寿命和硫酸盐生成速率分别为10.60 ~ 31.75 d和0.02 ~ 0.04 μg m-3 h-1。本文章由计算机程序翻译,如有差异,请以英文原文为准。
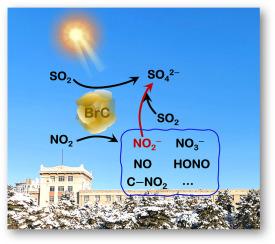
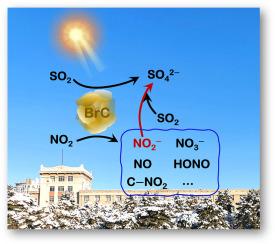
NO2-promoted heterogeneous photochemical oxidation of SO2 to sulfates on brown carbon
The role of NO2 in the heterogeneous photochemical formation of sulfates by SO2 uptake on typical brown carbon (BrC) species (Fluoranthene, FL; 4-(benzoyl) benzoic acid, 4–BBA; 3,7-dihydroxy-2-naphthoic acid, 3,7–DHNA) was systemically explored in a flow tube reactor with the infrared spectroscopy, ion chromatography as well as theoretical calculation. NO2 enhanced SO2 uptake and sulfate generation on BrC under irradiation, as shown by larger steady-state uptake coefficients of SO2 and sulfate formation rates. NO2− can be produced through the reduction of NO2 by the photogenerated electrons on BrC, and it was proven to be the trigger that promoted the oxidation of SO2 to sulfates. NO2− was more easily formed on FL, followed by 4−BBA and 3,7−DHNA, which can be evidenced by the energy barrier for NO2− production: FL (0.01 kJ mol−1) < 4−BBA (0.12 kJ mol−1) < 3,7−DHNA (1.33 kJ mol−1). SO2 lifetimes and sulfate formation rates during the reaction of SO2/NO2 with BrC were estimated to be 10.60–31.75 days and 0.02–0.04 μg m−3 h−1, respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


