使用绿色深共晶溶剂对水溶液中氢键网络的策略调制:对称超级电容器中增强电容性能的实验和计算见解
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
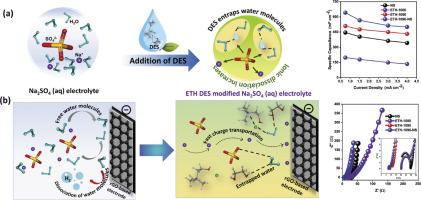
本研究探索了深度共晶溶剂(DESs)与水电解质的集成,为调节氢键网络、抑制水分解和提高电化学性能铺平了道路。提出了一种战略方法,通过加入氯化胆碱(ChCl)为基础的绿色DES,特别是ChCl和乙二醇(EG)(通常称为ethaline, ETH)来控制Na2SO4水溶液中的水解离。系统研究表明,在des -水混合物中不同的含水量(0%、40%、60%和90%)会影响其物理化学性质,包括电导率、粘度和电容性能。纯ETH (ETH-1000)的电导率在加入60%水(ETH-4060)时有所提高,但在加入90%水(ETH-1090)时由于稀释过度,电导率急剧下降。ETH-1090的粘度比ETH-1000低6倍,说明ETH组分之间的氢键因稀释而减弱。其中,ETH-1090与ETH-1000相比电容性能最高。此外,将ETH-1090 DES添加到Na2SO4水溶液电解质中,在扰乱原有氢键网络的同时,ETH-1090- ns基电解质中形成了新的独特类型的氢键。因此,这种策略调制抑制了水分解,提高了Na2SO4水溶液电解质的电化学稳定性,可用于rgo基SSC。这使得Na2SO4水溶液的电容性能显著提高,从445到645 mF cm-2,电导率更高,电化学电位窗口扩展到1.6 V。为了支持这些发现,进行了经典分子动力学(MD)模拟和量子化学计算。计算研究提供了ETH DES与水电解质之间分子相互作用的见解,揭示了氢键在操纵常规水电解质中的独特作用。优异的长期循环稳定性(在高电流密度下高达10,000次循环保持70%的电容)深刻地验证了开发的ETH-1090-NS电解质体系的鲁棒性。这项全面的研究强调了在原子水平上对传统水性电解质进行独特的DES修饰的潜力,以提高性能和推进能量存储系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Strategic modulation of hydrogen bonding networks in aqueous electrolytes using green deep eutectic solvents: Experimental and computational insights for enhanced capacitive performance in symmetric supercapacitors
This study explores the integration of deep eutectic solvents (DESs) with aqueous electrolytes, paving a pathway to modulate the hydrogen-bonding network, suppress water decomposition, and enhance electrochemical performance of a well-established electroactive reduced graphene oxide (rGO) based symmetrical supercapacitor (SSC). A strategic approach is proposed to control water dissociation in aqueous Na2SO4 electrolytes by incorporating a choline chloride (ChCl) based green DES, specifically ChCl and ethylene glycol (EG) (commonly referred to as ethaline, ETH). Systematic investigations reveal that varying water content (0 %, 40 %, 60 %, and 90 %) in the DES-water mixture affects its physicochemical properties, including conductivity, viscosity, and capacitive performance. The conductivity of neat ETH (ETH-1000) increased with up to 60 % water addition (ETH-4060) but dropped sharply to 90 % water content (ETH-1090) due to excessive dilution. The viscosity of ETH-1090 was six times lower than ETH-1000, reflecting hydrogen bond weakening among ETH components due to dilution. Among these, ETH-1090 demonstrated the highest capacitive performance compared to ETH-1000. Furthermore, the addition of ETH-1090 DES to the aqueous Na2SO4 electrolyte simultaneously disturbed the pre-existing hydrogen bonding network while fostering new and unique types of hydrogen bonds in ETH-1090-NS based electrolyte. Thus, this strategic modulation suppressed water decomposition and enhanced the electrochemical stability of aqueous Na2SO4 electrolyte for application in rGO-based SSC. This leads to a significant increase in the capacitive performance of aqueous Na2SO4 electrolyte from 445 to 645 mF cm-2 with greater conductivity and extended electrochemical potential window up to 1.6 V. To support these findings, classical molecular dynamics (MD) simulations and quantum chemical calculations were conducted. Computational study provides insights into molecular interactions between ETH DES and aqueous electrolyte, revealing the unique role of hydrogen bonds in manipulating the conventional aqueous electrolyte. The excellent long-term cyclic stability (70 % retention of capacitance up to 10,000 cycles at high current density) is profoundly validating the robustness of the developed ETH-1090-NS based electrolyte system. This comprehensive study highlights the potential of unique DES based modifications of conventional aqueous electrolytes at the atomic level to enhance performance and advance energy storage systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


