SnO2电极上的脉冲电催化提高CO2电还原过程中甲酸盐的选择性和活性
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
氧化锡(SnO2)被认为是电催化CO2还原(CO2R)生成甲酸转化的候选催化剂。然而,在高电流密度下,SnO2自还原为金属锡,导致甲酸盐选择性不可避免地急剧下降。本文通过对SnO2前驱体的脉冲电催化合成了一种基于SnO2的催化剂(pull -SnO2)。由于能够保持高氧化价态并促进氧空位的形成,在600 mA cm−2的高电流密度下,pull - sno2表现出90%的甲酸盐选择性,显著高于通过恒电位电催化获得的传统锡基催化剂(81%和100 mA cm−2)。原位拉曼光谱、动力学同位素效应、循环伏安法和理论计算表明,SnO2的高氧化态促进了CO2分子的活化,氧空位增强了水的解离,从而加速了质子耦合电子转移过程,降低了*OCHO中间体生成的自由能。此外,在CO2R过程中,已识别的具有适当覆盖的吸附羟基(*OH)也促进了*OCHO的形成,进一步使*OCHO的形成更加有利于能量。结果表明,pull - sno2催化剂在保持良好活性和稳定性的同时,对CO2R的生成具有超强的选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
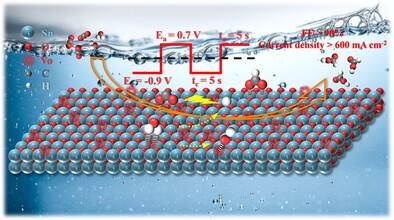
Pulsed Electrocatalysis on SnO2 Electrodes for Boosting Formate Selectivity and Activity during CO2 Electroreduction
Tin oxide (SnO2) is considered a candidate catalyst for the electrocatalytic CO2 reduction (CO2R) to formate conversion. However, the self-reduction of SnO2 to metallic Sn at high current densities leads to an unavoidable sharp decrease in formate selectivity. Herein, a SnO2-based catalyst (Pul-SnO2) is synthesized via pulsed electrocatalysis of SnO2 precursors. Due to the ability to maintain the high oxidation valence states and promote the formation of oxygen vacancies, Pul-SnO2 exhibited a high formate selectivity of 90% at a high current density of 600 mA cm−2, significantly higher than that of a conventional Sn-based catalyst (81% and 100 mA cm−2) obtained via constant potential electrocatalysis. The in situ Raman spectra, kinetic isotope effect, cyclic voltammetry, and theoretical calculations demonstrated that the high oxidation states of SnO2 promote CO2 molecules activation and the oxygen vacancies enhance water dissociation, thereby accelerating the proton-coupled electron transfer process to reduce the free energy of *OCHO intermediate generation. Moreover, the identified adsorbed hydroxyls (*OH) with suitable coverage during CO2R also promote the *OCHO formation and further make the formation of *OCHO more energy-favorable. As a result, the Pul-SnO2 catalyst showed a super selectivity in CO2R to formate, while maintaining excellent activity and stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


