PET废塑料电解升级回收的节能析氢研究
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
聚对苯二甲酸乙二醇酯(PET)电催化重整是一种可持续的塑料垃圾处理方法。然而,大多数电催化剂仅对PET水解产物(乙二醇,EG)的氧化有活性,对PET的分解没有活性。在此,我们提出了一种由钼多元金属氧酸盐(H3PMo12O40, PMo12)混合酸体系催化的PET分解和电解升级循环路线,该路线可以将废弃PET转化为对苯二甲酸(TPA)和氢燃料。PMo12是一种高效的PET水解催化剂,即使在温和的条件下(<100°C, 1atm,低酸浓度<;2.5 mol L−1)。它同时将水解产物EG氧化为甲酸(FA),促进PET连续水解并导致PET几乎完全转化(~ 100%)。PMo12催化剂还通过耦合电解过程作为电载体回收。氢气的生产能耗较低,为2.09 kW h N m−3,仅为水电解所需能量的50.6%。Aspen建模和生命周期评估(LCA)分析表明,PET电解升级回收路线具有巨大的工业潜力和减少碳足迹。本文章由计算机程序翻译,如有差异,请以英文原文为准。
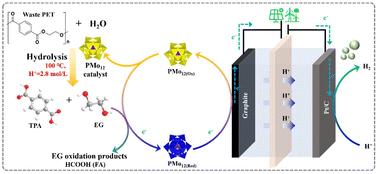
Electrolytic upcycling of PET waste plastics for energy-efficient hydrogen evolution†
Electrocatalytic reforming of polyethylene terephthalate (PET) is a sustainable way to treat plastic waste. However, most of the electrocatalysts are only active for PET hydrolyzed product (ethylene glycol, EG) oxidation, not for PET decomposition. Herein, we present a PET decomposition and electrolytic upcycling route catalyzed by a molybdic polyoxometalate (H3PMo12O40, PMo12) mixed acid system, which can convert waste PET into terephthalic acid (TPA) and hydrogen fuel. PMo12 is an efficient PET hydrolysis catalyst even under mild conditions (<100 °C, 1 atm, and low acid concentration <2.5 mol L−1). It simultaneously oxidizes the hydrolyzed product, EG, to formic acid (FA), promoting continuous PET hydrolysis and leading to a near-complete conversion of PET (∼100%). The PMo12 catalyst also acts as an electro-carrier recycled through a coupled electrolysis process. H2 is produced with a low energy consumption of 2.09 kW h N m−3, which is only 50.6% of the energy required for water electrolysis. Aspen modeling and Life Cycle Assessment (LCA) analysis show great industrial potential and a reduced carbon footprint of the PET electrolytic upcycling route.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


