超高真空下非晶冰薄膜中CO2笼形物水合物笼的解离与重组
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在无定形冰中形成笼形水合物(CHs)具有重要的天体化学意义。CO2通常在真空条件下由无定形水-CO2冰混合物成核形成I (sI) CH结构。本研究首次报道了结构II (sII) CO2 CH,其中CO2在超高真空(UHV)条件下在二甲醚(DME)或四氢呋喃(THF)存在下占据大笼。将CO2:水混合物在二甲醚上连续气相沉积,温度为10 K,并将混合冰膜热退火至130 K,制备了二甲醚-二氧化碳混合CHs。在二甲醚的sII - CH大笼形成过程中,预形成的sII -CO2 - CH小笼发生部分解离,同时形成新的sII -CO2 - CH大笼。在THF-CO2混合CHs中也观察到类似的结果。此外,THF-DME-CO2的混合CHs在130 K退火时形成sII水合物。在130 K下长时间退火(37 h)导致混合CHs解离,释放CO2和二甲醚,增加THF - CH分数。这些观察结果突出表明,在相同条件下,与CO2和二甲醚相比,THF - CH具有更大的稳定性。这些发现增强了我们对模拟星际条件下混合CHs的结构动力学和形成机制的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
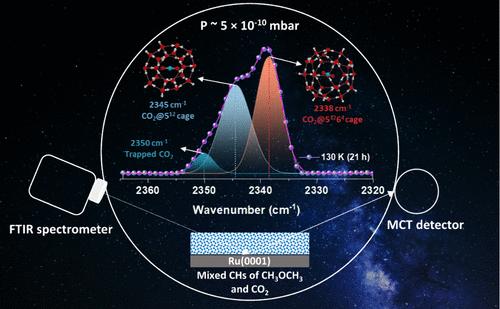
Dissociation and Reformation of CO2 Clathrate Hydrate Cages in Amorphous Ice Thin Film under Ultrahigh Vacuum
The formation of clathrate hydrates (CHs) within amorphous ice holds significant astrochemical importance. CO2 typically forms structure I (sI) CH under vacuum, upon nucleation from an amorphous water-CO2 ice mixture. This study presents the first report of structure II (sII) CO2 CH, where CO2 occupies large cages in the presence of dimethyl ether (DME) or tetrahydrofuran (THF) under ultrahigh vacuum (UHV) conditions. Mixed CHs of DME-CO2 were prepared by sequential vapor phase deposition of CO2:water mixture over DME at 10 K and thermally annealing this mixed ice film to 130 K. During the formation of large cages of sII CH of DME, partial dissociation of the preformed small cage of sI of CO2 CH occurs along with the formation of new large cages of sII CO2 CH. Similar results were observed for THF-CO2 mixed CHs. Additionally, mixed CHs of THF-DME-CO2 formed sII hydrates when annealed at 130 K. Prolonged annealing (37 h) at 130 K led to the dissociation of mixed CHs, releasing CO2 and DME and increasing the THF CH fraction. These observations highlight the greater stability of THF CH compared with those of CO2 and DME under identical conditions. These findings enhance our understanding of the structural dynamics and formation mechanisms of mixed CHs under simulated interstellar conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


