钯催化苯乙烯氧烯烃制氟烯丙基醚的研究
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
烯丙醚是一种有价值的骨架,广泛存在于天然产物中。它们也是重要的合成中间体,特别是作为Tsuji-Trost反应的前体。然而,尽管在生物化学中已很好地认识到氟烯烃是酰胺的同分异构体,但它们的氟化类似物仍未得到充分开发。在此,我们公开了一个便利的pd催化平台,通过与醇和2,2-二溴-2-氟酰胺化合物的氧烯烃化,构建氟烯丙基醚,涉及C = C键形成过程。该方案在温和的反应条件下与广泛的芳香烃具有良好的相容性,包括改性生物分子和含有苯乙烯基序的药物衍生物。机理研究表明,氟烷基自由基和π-烯丙基钯配合物作为关键中间体在这一转化过程中起关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
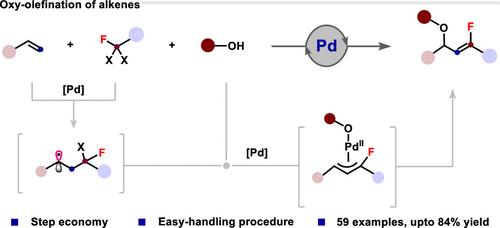
Palladium-Catalyzed Oxy-olefination of Styrenes toward Fluoroallyl Ethers
Allyl ethers are valuable skeletons that occur widely in natural products. They also serve as important synthetic intermediates, particularly as precursors in Tsuji–Trost reactions. However, their fluorinated analogues remain underexplored, despite the good recognition of fluoroalkenes as isosteres of amides in biological chemistry. Herein, we disclosed an expedient Pd-catalytic platform to construct fluoroallyl ethers via oxy-olefination of easily available alkenes with alcohols and 2,2-dibromo-2-fluoroamide compounds involving a C═C bond formation process. The protocol demonstrates excellent compatibility with a broad range of aromatic alkenes under mild reaction conditions, including modified biomolecules and drug derivatives containing styrene motifs. Mechanistic investigations suggest that fluoroalkyl radicals and the π-allyl palladium complex play pivotal roles as key intermediates in this transformation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


