MnFe2O4/g-C3N4催化剂在大pH范围内高效去除废水中的吡虫啉
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
采用非均相电fenton (HEF)法制备了MnFe2O4/g-C3N4复合材料,用于高效去除废水中的吡虫啉(IMD)。通过XRD、FTIR、Raman、N2吸附-解吸分析、SEM-EDS和XPS对所制备的催化剂进行了表征,证明MnFe2O4与g-C3N4成功结合。此外,利用电化学电池进行HEF脱除IMD,考察了MnFe2O4/g-C3N4的催化性能。在MnFe2O4/g- c3n4配比为1:2,(1:2)MnFe2O4/g- c3n4用量为0.3 g L−1,电流密度为10 mA cm−2,初始pH为5.5(自然pH)的条件下,电解60 min后,IMD的分解率为99.7 %。这些结果证实了该系统在0.107 kWh g−1的最低能耗(EC)下具有优异的去除性能。延长处理时间3 h, TOC去除率达到87.6% %。HEF工艺的矿化电流效率(MCE)提高了14.5 %,EC降低了0.84 kWh g−1 TOC。猝灭实验表明,羟基自由基(•OH)是IMD降解的主要活性物质。此外,MnFe2O4/g-C3N4的HEF在不同的水基质中表现出优异的催化效果,在60 min后,对实际废水的IMD去除率达到84.5 %。本研究评价了(1:2)MnFe2O4/g-C3N4催化剂在5次循环中的可回收性和稳定性,并通过XRD、FTIR和XPS等多种表征分析证实了这一点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
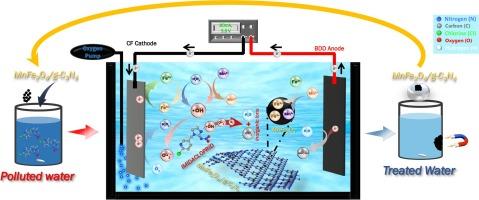
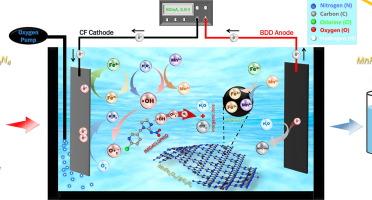
High-efficiency removal of imidacloprid from wastewater by heterogeneous electro-Fenton process using MnFe2O4/g-C3N4 catalyst in a wide range of pH
MnFe2O4/g-C3N4 composites were prepared for the high-efficiency removal of Imidacloprid (IMD) in wastewater via Heterogeneous Electro-Fenton (HEF) process. The prepared catalysts were characterized by XRD, FTIR, Raman, N2 adsorption–desorption analysis, SEM-EDS, and XPS, which proved that the MnFe2O4 was successfully combined with g-C3N4. Moreover, IMD removal by the HEF process using an electrochemical cell was carried out to examine the catalytic performance of the MnFe2O4/g-C3N4. 99.7 % of IMD was decomposed after 60 min of electrolysis under the optimal conditions: the ratio MnFe2O4/g-C3N4 was 1:2, the dose of (1:2) MnFe2O4/g-C3N4 was 0.3 g L−1, the current density was 10 mA cm−2 and the initial pH was 5.5 (natural pH). These results confirmed the excellent removal performance of the system at the lowest Energy Consumption (EC) of 0.107 kWh g−1. Prolonged treatment time of 3 h achieved 87.6 % TOC removal. The HEF process demonstrated an improved Mineralization Current Efficiency (MCE) of 14.5 % and a reduced EC of 0.84 kWh g−1 TOC. Quenching experiments exhibited the hydroxyl radicals (•OH) as the primary active species for IMD degradation. Moreover, the HEF with MnFe2O4/g-C3N4 showed excellent catalytic effectiveness in different water matrices, reaching 84.5 % IMD removal in real wastewater after 60 min. This study evaluates the recyclability and the stability of (1:2) MnFe2O4/g-C3N4 catalyst during 5 cycles, which was confirmed by various characterization analyses of the used catalyst such as XRD, FTIR and XPS measurements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


