非水介质中Cu电沉积动力学和热力学控制对Cu离子的可调稳定
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在这项工作中,Cu+离子在含有TFSI -和Cl -阴离子的非水电解质中以二甘醇为溶剂实现了显著的稳定。当没有Cl -时,由于Cu+在二酶中的稳定常数很低,Cu+发生稳定化。然而,在相对较低的Cl -浓度(即相对于Cu2+等摩尔浓度)存在下,Cu+和Cu2+都形成非常稳定的Cu2+/+[Cl -]络合物,导致Cu+/0氧化还原对相对于更容易还原的Cu+[TFSI -][Cl -]络合物产生~ 1200 mV的负位移。这些结果表明,相对于水,非水溶剂较低的溶剂化能和较宽的电化学稳定窗口使阴离子在指导络合行为方面发挥更重要的作用──为反应中间体的(电)化学稳定提供了新的可能性,并突出了非水体系中电解质设计的大量未开发机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
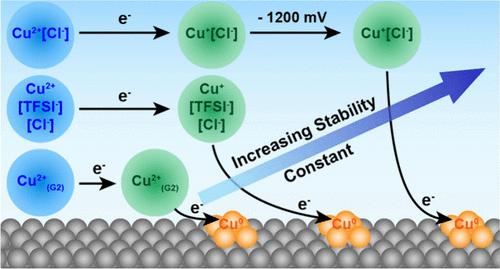
Tunable Stabilization of Cuprous Ions via Kinetic and Thermodynamic Control of Cu Electrodeposition in Non-Aqueous Media
In this work, significant stabilization of Cu+ ions is achieved in non-aqueous electrolytes containing TFSI– and Cl– anions with diglyme as solvent. When Cl– is absent, stabilization of Cu+ occurs due to the very low stability constant of Cu2+ in diglyme. However, in the presence of relatively low Cl– concentrations (i.e., equimolar relative to Cu2+), both Cu+ and Cu2+ form extremely stable Cu2+/+[Cl–] complexes resulting in a ∼1200 mV negative shift of the Cu+/0 redox couple relative to that of more easily reduced Cu+[TFSI–][Cl–] complexes. These results suggest that the lower solvation energies and wider electrochemical stability windows of non-aqueous solvents relative to water enable anions to play a much more significant role in guiding complexation behavior─providing new possibilities for (electro)chemical stabilization of reactive intermediates and highlighting the wealth of unexplored opportunities for electrolyte design in non-aqueous systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


