蛋白固定化激发溶酶体破坏高效核药物递送
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
肿瘤细胞的高代谢和过度生长导致肿瘤微环境(tumor microenvironment, TME)的形成和细胞内溶酶体活性的增强,从而消除化疗药物。因此,设计针对TME和靶向肿瘤细胞溶酶体的纳米载体是提高药物特异性和利用效率的最佳策略。在此,受蛋白质固定化的启发,开发了一种双响应的超分子纳米药物FPA/DOX,专门针对TME和肿瘤细胞溶酶体。在缺氧和酸性反应中,FPA/DOX通过蛋白质共价固定反应(一种亚酰化缩合反应)暴露与溶酶体蛋白上的氨基结合的苯甲醛基团,导致蛋白质变性和失活,并诱导溶酶体膜透性(LMP)。这种LMP过程不仅触发溶酶体依赖性细胞死亡(LDCD),而且还促进释放的DOX快速易位到细胞核中。体外实验表明,FPA/DOX对肿瘤细胞的毒性是游离DOX的4.2倍。此外,体内研究证实FPA/DOX具有较高的生物安全性,肿瘤抑制率高达95.27%。综上所述,由蛋白质固定化激发的溶酶体破坏已经开创了一种治疗肿瘤的方法,并在生物医学应用中具有巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
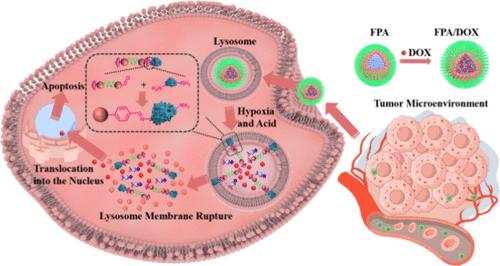
Protein Immobilization Inspired Lysosomal Disruption for Efficient Nuclear Drug Delivery
The high metabolism and excessive growth of tumor cells result in the development of a tumor microenvironment (TME) and enhanced lysosomal activity within the cells, which can eliminate chemotherapeutics. Consequently, the design of nanocarriers that respond to TME and target tumor cell lysosomes represents an optimal strategy to enhance drug specificity and utilization efficiency. Herein, inspired by protein immobilization, a dual-responsive supramolecular nanomedicine FPA/DOX is developed for specifically targeting the TME and tumor cell lysosomes. Upon hypoxia and acidic response, FPA/DOX exposes benzaldehyde groups that engage with amino groups on lysosomal proteins by protein covalent immobilization reaction─an imidization condensation reaction, leading to protein denaturation and inactivation and inducing lysosomal membrane permeabilization (LMP). This LMP process not only triggers lysosomal-dependent cell death (LDCD) but also facilitates the rapid translocation of released DOX into the cell nucleus. In vitro experiments have demonstrated that the tumor cell toxicity of FPA/DOX is 4.2 times that of free DOX. Additionally, in vivo studies have verified the high biosafety of FPA/DOX, with a remarkable tumor inhibition rate of 95.27%. In summary, the lysosomal disruption inspired by protein immobilization has pioneered an approach for tumor treatment and holds great potential in biomedical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


