fe基非晶合金中FeSx层的原位形成:硫化物层形成机理及催化活性的提高
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
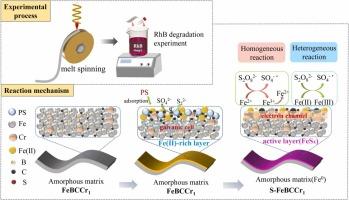
硫化零价铁(S-ZVI)已被证明是改善ZVI表面钝化和加速电子转移的可靠方法。然而,硫化铁层的消耗导致其寿命较短。在本研究中,成功制备了硫化FeBCCr1非晶带,在较宽的初始pH范围(2.0-10.0)内有效激活过硫酸盐(PS)降解RhB,结果显色率接近100%,总有机碳去除率达到66.3%。根据实验结果和吸附现象,提出了一种表面介导的非均相活化机理。在非晶带表面生成的原位“自重构”硫化铁层(FeSx)作为助催化剂,促进电子从基体Fe0转移,不断加速Fe3+/Fe2+的循环。降解过程中不断生成的FeSx防止了层的老化,保证了FeBCCr1非晶带的催化活性。更重要的是,Fe3+的共存极大地提高了硫化层的稳定性、电子利用效率和材料的可重复使用性,这是由于FeBCCr1/PS/Fe3+体系中Fe3+通过电荷转移转化为Fe2+的效率提高和Fe0的低消耗。本研究为FeBCCr1/PS体系中FeSx共催化剂层的设计提供了新的策略,为宏观制备硫化铁基非晶带提供了有效途径,为零价铁在环境领域的应用奠定了坚实的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
In situ formation of FeSx layer on Fe-based amorphous alloys: Improvement of catalytic activity and mechanism of sulfide layer formation
Sulfidated zero-valent iron (S-ZVI) has been demonstrated as a reliable approach for improving the surface passivation and accelerating electron transfer of ZVI. However, consumption of the iron sulfide layer resulted in a short lifespan. In this study, sulfidated FeBCCr1 amorphous ribbons were successfully fabricated to effectively activate the persulfate (PS) for RhB degradation over a wide initial pH range (2.0–10.0), with a result of nearly 100 % of the color and 66.3 % of the total organic carbon removal efficiency. Based on the experiment results and adsorption phenomena, a surface-mediated heterogeneous activation mechanism was proposed. The in situ “self-reconstruction” iron sulfide layer (FeSx) generated on the surface of the amorphous ribbon acted as a cocatalyst to promote electron transfer from the matrix Fe0 and continually accelerated the circulation of Fe3+/Fe2+. The continuous generation of FeSx during degradation prevented the layer aging and ensured the catalytic activity of the FeBCCr1 amorphous ribbons. More importantly, the co-existence of Fe3+ dramatically improved the stability of sulfide layer, electron utilization efficiency and material reusability, which was attributed to the improved conversion of Fe3+ to Fe2+ through charge transfer and the low consumption of Fe0 in FeBCCr1/PS/Fe3+ system. This work provides a new strategy for designing FeSx co-catalyst layer in FeBCCr1/PS system and provides an effective approach for macroscopic preparation of sulfidated Fe-based amorphous ribbons, establishing a solid foundation for the application of zero valent iron in the environmental field.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


