金丝桃苷调节胆汁酸和脂肪酸代谢,是非酒精性脂肪性肝病的潜在治疗方法
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
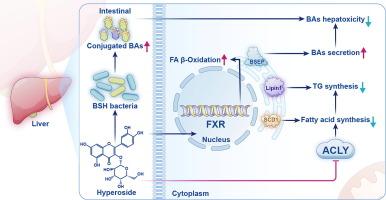
非酒精性脂肪性肝病(NAFLD)是一种多因素慢性疾病,需要系统有效的治疗方法。中药衍生的多效治疗药物越来越被认为是NAFLD干预的有希望的替代方法。金丝桃苷是一种天然的黄酮苷,存在于菟丝子、连翘和山楂中,已被证明能有效缓解大鼠NAFLD。然而,金丝桃苷缓解NAFLD的潜在机制尚不清楚。目的探讨金丝桃苷干预NAFLD进展的具体机制。方法采用高脂饮食法诱导大鼠NAFLD模型。综合分析,包括基于质谱的脂质组学、基于tmt的蛋白质组学、16S rRNA测序和胆汁酸靶向代谢组学,用于鉴定显著改变的代谢物和蛋白质。采用Western blotting、分子对接、等温滴定量热等方法分析其直接作用靶点。结果金丝桃苷激活法脂类 X 受体(FXR),促进脂肪酸氧化和胆汁酸从肝脏排出。此外,金丝桃苷抑制肝脏ATP柠檬酸裂解酶(ACLY),并与活化的FXR协同作用,抑制新生脂肪生成。金丝桃苷还能抑制与胆盐水解酶(BSH)活性相关的肠道微生物,从而促进回肠胆汁酸(BAs)的产生,尤其是共轭胆汁酸,从而降低内源性胆汁酸的肝毒性。结论金丝桃苷通过FXR和ACLY调节脂肪酸和胆汁酸代谢,减轻NAFLD的影响,可能成为治疗NAFLD的多效候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hyperoside modulates bile acid and fatty acid metabolism, presenting a potentially promising treatment for non-alcoholic fatty liver disease
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a multifactorial chronic condition that requires a systematic approach for effective management. Multi-effect therapeutic drugs derived from traditional Chinese medicine are increasingly being recognized as promising alternatives for NAFLD intervention. Hyperoside, a natural flavone glycoside found in Cuscuta chinensis Lam, Forsythia suspensa, and Crataegus pinnatifida Bge, has been shown to effectively mitigate NAFLD in rats. However, the underlying mechanism through which hyperoside alleviates NAFLD remains unclear.Objective
This study aims to explore the specific mechanisms by which hyperoside intervenes in the progression of NAFLD.Methods
In this study, a high-fat diet was used to induce the NAFLD model in rats. An integrated analysis, including mass spectrometry-based lipidomics, TMT-based proteomics, 16S rRNA sequencing, and bile acid-targeted metabolomics, was employed to identify significantly altered metabolites and proteins. Western blotting, molecular docking, and isothermal titration calorimetry were conducted to analyze the direct targets of action.Results
The results indicate that hyperoside activates farnesoid X receptor (FXR), promoting fatty acid oxidation and the efflux of bile acids from the liver. Additionally, hyperoside inhibits hepatic ATP citrate lyase (ACLY) and works synergistically with activated FXR to suppress de novo lipogenesis. Hyperoside also inhibits intestinal microbes linked to bile-salt hydrolase (BSH) activity, which enhances the production of ileal bile acids (BAs), particularly conjugated BAs, thus reducing the liver toxicity of endogenous BAs.Conclusion
Our findings suggest that hyperoside alleviates NAFLD by modulating fatty acid and bile acid metabolism through FXR and ACLY, suggesting its potential as a multi-effect candidate drug for the treatment of NAFLD.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


