一种基于tRNA-gRNA阵列的CRISPR系统,用于高效、精确地对里氏木霉进行无标记多基因编辑,以提高纤维二糖的产量
IF 6.2
1区 农林科学
Q1 AGRICULTURAL ENGINEERING
引用次数: 0
摘要
纤维素二糖可被纤维素二糖磷酸化酶催化生成葡萄糖-1-磷酸,葡萄糖-1-磷酸再合成淀粉。与葡萄糖合成淀粉相比,这是一个更节能的过程。丝状真菌里氏木霉能够分泌大量的纤维素酶,将纤维素降解为纤维素二糖,然后继续释放葡萄糖作为最终产物。本研究以T. reesei菌株PXK1为基础,通过连续激活XyrlA824V和删除ku70基因,提高了同源重组效率,构建了高效、无标记、可循环的多基因编辑系统。包含CRISPR-Cas9框架、基于ama1的质粒和固有tRNA加工机制的基因编辑系统对1个、2个和3个基因的编辑效率分别高达100 %、82 %和67 %。11株β-葡萄糖苷酶(BG)单缺失菌株和数株β-葡萄糖苷酶双、三重缺失菌株。评估了BG缺失对纤维二糖生产的影响。与菌株PXK1相比,菌株Δcel3a的纤维二糖产量提高了128 %,而累积缺失两个BG基因的菌株比Δcel3a的纤维二糖产量有效提高了12 %。本文章由计算机程序翻译,如有差异,请以英文原文为准。
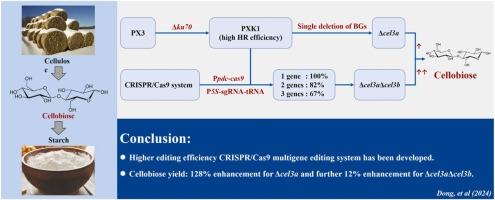
A tRNA-gRNA array-based CRISPR system for efficient and precise marker-free multiple gene editing of Trichoderma reesei to enhance cellobiose production
Cellobiose can be catalyzed by cellobiose phosphorylase to produce glucose-1-phosphate, which is then further synthesized into starch. This is a more energy efficient process compared to starch synthesis from glucose. The filamentous fungi Trichoderma reesei has the ability to secrete large amounts of cellulases to degrade cellulose to cellobiose, then continue to release glucose as the end product. In this study, we developed a highly efficient, marker-free and recyclable multigene editing system based on a T. reesei strain PXK1 with consecutively activated XyrlA824V and deletion of ku70 gene with increased homologous recombination efficiency. The gene editing system which contained the CRISPR-Cas9 framework, the AMA1-based plasmid, and an intrinsic tRNA processing mechanism showed up to 100 %, 82 % and 67 % efficiency for editing one, two and three genes, respectively. Eleven single strains with single β-glucosidase (BG) deletion and then several strains with double and triple β-glucosidase deletions were obtained. The effect of BG deletions on cellobiose production were evaluated. The strain Δcel3a showed a 128 % increase in cellobiose yield compared to strain PXK1, and the strain with cumulative deletion of two BG genes can efficiently produce 12 % more cellobiose than Δcel3a.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial Crops and Products
农林科学-农业工程
CiteScore
9.50
自引率
8.50%
发文量
1518
审稿时长
43 days
期刊介绍:
Industrial Crops and Products is an International Journal publishing academic and industrial research on industrial (defined as non-food/non-feed) crops and products. Papers concern both crop-oriented and bio-based materials from crops-oriented research, and should be of interest to an international audience, hypothesis driven, and where comparisons are made statistics performed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


