变废为宝:放射性副产物诱导的铁(III)/铁(II)转化有效的铁下沉改善碘-131经动脉放射栓塞治疗肝肿瘤
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
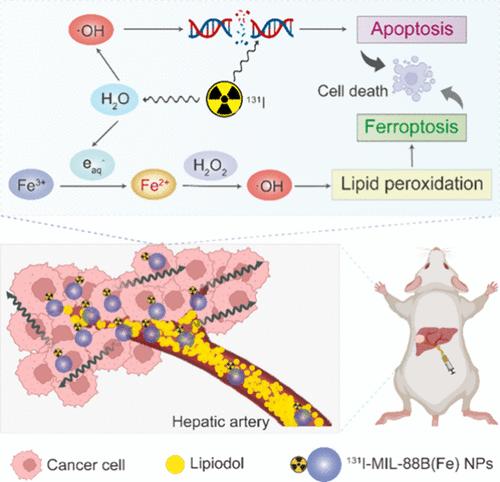
经动脉放射栓塞(TARE)是晚期肝癌的主要姑息性治疗方法。然而,其治疗效果经常受到放疗间期诱导的肿瘤细胞凋亡抵抗的阻碍。诱导多种细胞死亡模式为这一挑战提供了一个潜在的解决方案。铁凋亡是细胞凋亡的一种不同形式的细胞死亡,依赖于细胞内Fe2+介导的Fenton反应产生羟基自由基(·OH),并被认为是一种有前途的癌症治疗方法。在本研究中,我们将131I标记的铁基MIL-88B(Fe)纳米粒子(NPs)(简称131I-MIL-88B(Fe))与lipodol结合,合成了一种治疗性放射性核素碘-131 (131I)基TARE药物,以实现细胞凋亡-铁凋亡肿瘤的联合治疗。具体地说,脂醇和131I-MIL-88B(Fe) NPs的混合物通过肝动脉注射到肝脏肿瘤中。脂醇阻断肿瘤动脉血供,引起肿瘤组织坏死,而131I放射间期通过直接作用或通过水溶产生·OH间接作用损伤脱氧核糖核酸(DNA),导致肿瘤细胞凋亡。重要的是,水合电子(eaq -)作为水辐射分解的副产物,促进了MIL-88B(Fe) NPs中Fe3+向Fe2+的转化,增强了Fenton反应的功效,并引发了铁下沉。体外实验表明,与131I相比,131I- mil - 88b (Fe) NPs显著增强了铁中毒介导的肿瘤细胞死亡,这是由于131I诱导的Fe2+产生,从而增加了Fenton反应的催化活性。在原位N1S1肝肿瘤大鼠模型中,TARE加脂醇和131I-MIL-88B(Fe) NPs可诱导肿瘤细胞坏死、凋亡和铁上吊,改善治疗效果。本研究利用eq -促进Fe3+/Fe2+的有效转化,将废物转化为有价值的资源。这表明了多种治疗策略的创新整合,以增强TARE在肝癌治疗中的疗效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Turning Waste into Treasure: Radiation Byproduct-Induced Fe(III)/Fe(II) Conversion for Efficient Ferroptosis to Improve Iodine-131-Based Transarterial Radioembolization for Liver Tumors
Transarterial radioembolization (TARE) is a primary palliative treatment for advanced liver cancer. Nonetheless, its therapeutic efficacy is frequently hindered by resistance to tumor cell apoptosis induced by inter-radiotherapy. Induction of multiple cell death modalities provides a potential solution to this challenge. Ferroptosis, a distinct form of cell death from apoptosis, is dependent on the intracellular Fe2+-mediated Fenton reaction for the production of hydroxyl radicals (·OH) and is gaining recognition as a promising approach for cancer treatment. In this study, we synthesized a therapeutic radionuclide iodine-131 (131I)-based TARE agent by combining 131I-labeled iron-based MIL-88B(Fe) nanoparticles (NPs) (abbreviated as 131I-MIL-88B(Fe)) with Lipiodol to achieve a combined apoptosis–ferroptosis tumor therapy. Specifically, a mixture of Lipiodol and 131I-MIL-88B(Fe) NPs was injected into the liver tumors through the hepatic artery. Lipiodol blocks the arterial blood supply of the tumor, causing tumor tissue necrosis, whereas 131I inter-radiotherapy damages deoxyribonucleic acid (DNA) through direct action or indirectly via the production of ·OH through H2O radiolysis, leading to tumor cell apoptosis. Importantly, hydrated electrons (eaq–), a byproduct of H2O radiolysis, promoted the conversion of Fe3+ to Fe2+ in MIL-88B(Fe) NPs, enhancing the efficacy of the Fenton reaction and triggering ferroptosis. In vitro experiments demonstrated that compared to 131I alone, 131I-MIL-88B(Fe) NPs significantly enhanced ferroptosis-mediated tumor cell death due to 131I-induced Fe2+ production, which increased catalytic activity in the Fenton reaction. In a rat model bearing orthotopic N1S1 liver tumors, TARE with Lipiodol and 131I-MIL-88B(Fe) NPs induced tumor cell necrosis, apoptosis, and ferroptosis, resulting in improved therapeutic outcomes. This study leverages eaq– to facilitate Fe3+/Fe2+ conversion for efficient ferroptosis, turning waste into a valuable resource. This demonstrated the innovative integration of multiple treatment strategies to augment the efficacy of TARE in liver cancer therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


