NaCl-KCl熔盐体系中低温电化学生产硼化钍的研究
IF 3.3
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
采用熔盐电化学方法,在NaCl-KCl-ThF₄-KBF₄体系中,在973 K下合成了硼化钍。形成的硼化物类型受电流密度和B/Th摩尔比的影响。较低的B/Th比有利于ThB₄的形成,较高的B/Th比有利于ThB₆的形成。这种方法为钍化合物合成提供了一种绿色、低温的替代方法,支持可持续的TMSR燃料回收。本文章由计算机程序翻译,如有差异,请以英文原文为准。
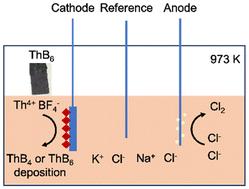
Low-temperature electrochemical production of thorium borides in NaCl-KCl molten salt systems†
Thorium borides were synthesized via molten-salt electrochemistry in a NaCl-KCl-ThF4-KBF4 system at 973 K. The type of boride formed is influenced by the current density and B/Th molar ratio. Lower B/Th ratios favor ThB4, while higher ratios promote ThB6 formation. This method provides a green, low-temperature alternative for thorium compound synthesis, supporting sustainable TMSR fuel recycling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


